Abstract
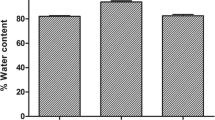
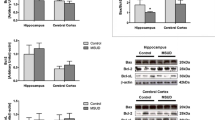
Maple syrup urine disease (MSUD) is an inborn error of metabolism caused by a severe deficiency in the activity of the branched-chain α-keto acid dehydrogenase complex, leading to accumulation of the branched-chain amino acids (BCAA) leucine, isoleucine, and valine. Infections have a significant role in precipitating acute metabolic decompensation in patients with MSUD; however, the mechanisms underlying the neurotoxicity in this disorder are poorly understood. In this study, we subjected rats to the coadministration of lipopolysaccharide (LPS), which is a major component of gram-negative bacteria cell walls, and high concentrations of BCAA (H-BCAA) to determine their effects on the permeability of the blood–brain barrier (BBB) and on the levels of matrix metalloproteinases (MMP-2 and MMP-9). Our results demonstrated that the coadministration of H-BCAA and LPS causes breakdown of the BBB and increases the levels of MMP-2 and MMP-9 in the hippocampus of these rats. On the other hand, examination of the cerebral cortex of the 10- and 30-day-old rats revealed a significant difference in Evan’s Blue content after coadministration of H-BCAA and LPS, as MMP-9 levels only increased in the cerebral cortex of the 10-day-old rats. In conclusion, these results suggest that the inflammatory process associated with high levels of BCAA causes BBB breakdown. Thus, we suggest that BBB breakdown is relevant to the perpetuation of brain inflammation and may be related to the brain dysfunction observed in MSUD patients.






Similar content being viewed by others
References
Chuang DT, Shih VE (2001) Maple syrup urine disease (branchedchain ketoaciduria). In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 1971–2005
Snyderman SE, Norton PM, Roitman E, Holt LE Jr (1964) Maple syrup urine disease, with particular reference to dietotherapy. Pediatrics 34:454–472
Gjedde A, Crone C (1983) Biochemical modulation of blood–brain barrier permeability. Acta Neuropathol Suppl (Berl) 8:59–74
Smith QR, Takasato Y (1986) Kinetics of amino acid transport at the blood–brain barrier studied using an in situ brain perfusion technique. Ann N Y Acad Sci 481:186–201
Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM (1999) Selective expression of the large neutral amino acid transporter at the blood–brain barrier. Proc Natl Acad Sci U S A 96:12079–12084
Killian DM, Chikhale PJ (2001) Predominant functional activity of the large, neutral amino acid transporter (LAT1) isoform at the cerebrovasculature. Neurosci Lett 306:1–4
Kamei A, Takashima S, Chan F, Becker LE (1992) Abnormal dendritic development in maple syrup urine disease. Pediatr Neurol 8:145–147
Araújo P, Wassermann GF, Tallini K et al (2001) Reduction of large neutral amino acid levels in plasma and brain of hyperleucinemic rats. Neurochem Int 38:529–537
Zinnanti WJ, Lazovic J, Griffin K et al (2009) Dual mechanism of brain injury and novel treatment strategy in maple syrup urine disease. Brain 132:903–918
Prensky AL, Moser HW (1966) Brain lipids, proteolipids, and free amino acids in maple syrup urine disease. J Neurochem 13:863–874
Dodd PR, Williams SH, Gundlach AL et al (1992) Glutamate and gamma-aminobutyric acid neurotransmitter systems in the acute phase of maple syrup urine disease and citrullinemia encephalopathies in newborn calves. J Neurochem 59:582–590
Howell RK, Lee M (1963) Influence of a-keto acids on the respiration of brain in vitro. Proc Soc Exp Biol Med 113:660–663
Danner DJ, Elsas LJ (1989) Disorders of branched chain amino acid and keto acid metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic basis of inherited disease. McGraw-Hill, New York, pp 671–692
Pilla C, Cardozo RF, Dutra-Filho CS, Wyse AT, Wajner M, Wannmacher CM (2003) Creatine kinase activity from rat brain is inhibited by branched-chain amino acids in vitro. Neurochem Res 28:675–679
Sgaravati AM, Rosa RB, Schuck PF et al (2003) Inhibition of brain energy metabolism by the a-keto acids accumulating in maple syrup urine disease. Biochim Biophys Acta 1639:232–238
Ribeiro CA, Sgaravatti AM, Rosa R et al (2008) Inhibition of brain energy metabolism by the branched-chain amino acids accumulating in maple syrup urine disease. Neurochem Res 33:114–124
Amaral AU, Leipnitz G, Fernandes CG, Seminotti B, Schuck PF, Wajner M (2010) Alpha-ketoisocaproic acid and leucine provoke mitochondrial bioenergetic dysfunction in rat brain. Brain Res 1324:75–84
de Franceschi ID, Rieger E, Vargas AP et al (2013) Effect of leucine administration to female rats during pregnancy and lactation on oxidative stress and enzymes activities of phosphoryltransfer network in cerebral cortex and hippocampus of the offspring. Neurochem Res 38:632–643
Jouvet J, Rustin P, Taylor DL et al (2000) Branched chain amino acids induce apoptosis in neural cells without mitochondrial membrane depolarization or cytochrome c release: implications for neurological impairment associated with maple syrup urine disease. Mol Biol Cell 11:1919–1932
Bridi R, Araldi J, Sgarbi MB et al (2003) Induction of oxidative stress in rat brain by the metabolites accumulating in maple syrup urine disease. Int J Dev Neurosci 21:327–332
Bridi R, Latini A, Braun CA et al (2005) Evaluation of the mechanisms involved in leucine induced oxidative damage in cerebral cortex of young rats. Free Radic Res 39:71–79
Fontella FU, Gassen E, Pulrolni V et al (2002) Stimulation of lipid peroxidation in vitro in rat brain by the metabolites accumulating in maple syrup urine disease. Metab Brain Dis 17:47–54
Barschak AG, Sitta A, Deon M et al (2006) Evidence that oxidative stress in increased in plasma from pacients with maple syrup urine disease. Metab Brain Dis 21:279–286
Mescka C, Moraes T, Rosa A et al (2011) In vivo neuroprotective effect of l-carnitine against oxidative stress in maple syrup urine disease. Metab Brain Dis 26:21–28
Scaini G, de Rochi N, Jeremias IC et al (2012) Evaluation of acetylcholinesterase in an animal model of maple syrup urine disease. Mol Neurobiol 45:279–286
Scaini G, Comim CM, Oliveira GM et al (2013) Chronic administration of branched-chain amino acids impairs spatial memory and increases brain-derived neurotrophic factor in a rat model. J Inherit Metab Dis 36:721–730
Scaini G, Mello-Santos LM, Furlanetto CB et al (2013) Acute and chronic administration of the branched-chain amino acids decreases nerve growth factor in rat hippocampus. Mol Neurobiol. doi:10.1007/s12035-013-8447-1
Holecek M, Sprongl L, Skopec F, Andrýs C, Pecka M (1997) Leucine metabolism in TNF-alpha- and endotoxin-treated rats: contribution of hepatic tissue. Am J Physiol 273:E1052–E1058
Fong Y, Matthews DE, He W, Marano MA, Moldawer LL, Lowry SF (1994) Whole body and splanchnic leucine, phenylalanine, and glucose kinetics during endotoxemia in humans. Am J Physiol 266:R419–R425
Holecek M, Sprongl L, Tichý M, Pecka M (1998) Leucine metabolism in rat liver after a bolus injection of endotoxin. Metabolism 47:681–685
Marshall JR, Gracy RW, Kester MV (1979) Maple syrup urine disease: response to dietary modifications. J Am Osteopath Assoc 79:98
Banks WA, Erickson MA (2010) The blood–brain barrier and immune function and dysfunction. Neurobiol Dis 37:26–32
Mrak RE, Griffin WS (2001) Interleukin-1, neuroinflammation, and Alzheimer’s disease. Neurobiol Aging 22:903–908
Marcondes MC, Burdo TH, Sopper S et al (2007) Enrichment and persistence of virus-specific CTL in the brain of simian immunodeficiency virus-infected monkeys is associated with a unique cytokine environment. J Immunol 178:5812–5819
O’Connor JC, McCusker RH, Strle K, Johnson RW, Dantzer R, Kelley KW (2008) Regulation of IGF-I function by proinflammatory cytokines: at the interface of immunology and endocrinology. Cell Immunol 252:91–110
Craft S (2009) The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol 66:300–305
Reale M, Greig NH, Kamal MA (2009) Peripheral chemo-cytokine profiles in Alzheimer’s and Parkinson’s diseases. Mini-Rev Med Chem 9:1229–1241
Combrinck MI, Perry VH, Cunningham C (2002) Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Neuroscience 112:7–11
Jaeger LB, Dohgu S, Sultana R et al (2009) Lipopolysaccharide alters the blood–brain barrier transport of amyloid beta protein: a mechanism for inflammation in the progression of Alzheimer’s disease. Brain Behav Immun 23:507–517
Abbott NJ (2005) Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol 25:5–23
Rubin LL, Staddon JM (1999) The cell biology of the blood brain barrier. Annu Rev Neurosci 22:11–28
Gonzalez-Mariscal L, Betanzos A, Avila-Flores A (2000) MAGUK proteins: structure and role in the tight junction. Semin Cell Dev Biol 11:315–324
Rosenberg GA, Kornfeld M, Estrada M, Kelley RO, Liotta LA, Stetler-Stevenson WG (1992) TIMP-2 reduces proteolytic opening of blood–brain barrier by type-IV collagenase. Brain Res 576:203–207
Rosenberg GA, Estrada EY, Dencoff JE, Stetler-Stevenson WG (1995) Tumor necrosis factor-alpha-induced gelatinase B causes delayed opening of the blood–brain barrier: an expanded therapeutic window. Brain Res 703:151–155
Schoettle RJ, Kochanek PM, Magargee MJ, Uhl MW, Nemoto EM (1990) Early polymorphonuclear leukocyte accumulation correlates with the development of posttraumatic cerebral edema in rats. J Neurotrauma 7:207–217
Allan SM, Rothwell NJ (2005) Inflammation in central nervous system injury. Philos Trans R Soc Lond Biol Sci 358:1669–1677
Stahel PF, Shohami E, Younis FM et al (2000) Experimental closed head injury: analysis of neurological outcome, blood–brain barrier dysfunction, intra-cranial neutrophil infiltration, and neuronal cell death in mice deficient in genes for pro-inflammatory cytokines. J Cereb Blood Flow Metab 20:369–380
Lucas SM, Rothwell NJ, Gibson RM (2006) The role of inflammation in CNS injury and disease. Brit J Pharmacol 147:S232–S240
Erickson MA, Banks WA (2011) Cytokine and chemokine responses in serum and brain after single and repeated injections of lipopolysaccharide: multiplex quantification with path analysis. Brain Behav Immun 25:1637–1648
Bridi R, Fontella FU, Pulrolnik V et al (2006) A chemically-induced acute model of maple syrup urine disease in rats for neurochemical studies. J Neurosci Methods 155:224–230
Uyama O, Okamura N, Yanase M, Narita M, Kawabata K, Sugita M (1988) Quantitative evaluation of vascular permeability in the gerbil brain after transient ischemia using evans blue fluorescence. J Cereb Blood Flow Metab 8:282–284
Coimbra RS, Loquet G, Leib SL (2007) Limited efficacy of adjuvant therapy with dexamethasone in preventing hearing loss due to experimental pneumococcal meningitis in the infant rat. Pediatr Res 62:291–294
Belayev L, Busto R, Zhao W, Ginsberg MD (1996) Quantitative evaluation of blood–brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res 739:88–96
Kago T, Takagi N, Date I, Takenaga Y, Takagi K, Takeo S (2006) Cerebral ischemia enhances tyrosine phosphorylation of occludin in brain capillaries. Biochem Biophys Res Commun 339:1197–1203
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood–brain barrier. Neurobiol Dis 37:13–25
Cornford EM, Cornford ME (1986) Nutrient transport and the blood–brain barrier in developing animals. Fed Proc 45:2065–2072
Banos G, Daniel PM, Pratt OE (1978) The effect of age upon the entry of some amino acids into the brain, and their incorporation into cerebral protein. Dev Med Child Neurol 20:335–346
Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC (1998) Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke 29:1020–1030
Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, Chan PH, Park TS (2005) Leukocyte-derived matrix metalloproteinase-9 mediates blood–brain barrier breakdown and is pro-inflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol 289:H558–H568
Page-McCaw A, Ewald AJ, Werb Z (2007) Matrix metalloproteinases and the regulation of tissue remodeling. Nat Rev Mol Cell Biol 8:221–233
Rosenberg GA, Navratil M (1997) Metalloproteinase inhibition blocks edema in intracerebral hemorrhage in the rat. Neurology 48:921–992
Anthony DC, Bolton SJ, Fearn S, Perry VH (1997) Age-related effects of interleukin-1 beta on polymorphonuclear neutrophil-dependent increases in blood–brain barrier permeability in rats. Brain 120:435–444
Chandler S, Miller KM, Clements JM et al (1997) Matrix metalloproteinases, tumor necrosis factor and multiple sclerosis: an overview. J Neuroimmunol 72:155–161
Cossins JA, Clements JM, Ford J et al (1997) Enhanced expression of MMP-7 and MMP-9 in demyelinating multiple sclerosis lesions. Acta Neuropathol (Berl) 94:590–598
Kieseier BC, Kiefer R, Clements JM et al (1998) Matrix metalloproteinase-9 and -7 are regulated in experimental autoimmune encephalomyelitis. Brain 121:159–166
Umehara F, Okada Y, Fujimoto N, Abe M, Izumo S, Osame M (1998) Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in HTLV-1-associated myelopathy. J Neuropathol Exp Neurol 57:839–849
Yong VW, Krekoski CA, Forsyth PA, Bell R, Edwards DR (1998) Matrix metalloproteinases and diseases of the CNS. Trends Neurosci 21:75–80 [Review]
Leib SL, Leepert D, Clements J, Tauber MG (2000) Matrix metalloproteinases contribute to brain damage in experimental pneumococcal meningitis. Infect Immun 68:615–620
Rosenberg GA (2009) Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol 8:205–216
Dal-Pizzol F, Rojas HA, dos Santos EM et al (2013) Matrix metalloproteinase-2 and metalloproteinase-9 activities are associated with blood–brain barrier dysfunction in an animal model of severe sepsis. Mol Neurobiol. doi:10.1007/s12035-013-8433-7
Grossetete M, Phelps J, Arko L, Yonas H, Rosenberg GA (2009) Elevation of matrix metalloproteinases 3 and 9 in cerebrospinal fluid and blood in patients with severe traumatic brain injury. Neurosurgery 65:702–708
Fernandez-Patron C, Radomski MW, Davidge ST (1999) Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res 85:906–911
He S, Prasanna G, Yorio T (2007) Endothelin-1-mediated signaling in the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in astrocytes. Investig Ophthalmol Vis Sci 48:3737–3745
Szklarczyk A, Lapinska J, Rylski M, McKay RD, Kaczmarek L (2002) Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. J Neurosci 22:920–930
Michaluk P, Kaczmarek L (2007) Matrix metalloproteinase-9 in glutamatedependent adult brain function and dysfunction. Cell Death Differ 14:1255–1258
Dong X, Song YN, Liu WG, Guo XL (2009) Mmp-9, a potential target for cerebral ischemic treatment. Curr Neuropharmacol 7:269–275
Yin P, Yang L, Zhou HY, Sun RP (2011) Matrix metalloproteinase-9 may be a potential therapeutic target in epilepsy. Med Hypotheses 76:184–186
Ralay Ranaivo H, Hodge JN, Choi N, Wainwright MS (2012) Albumin induces upregulation of matrix metalloproteinase-9 in astrocytes via MAPK and reactive oxygen species-dependent pathways. J Neuroinflammation 9:68
Leppert D, Lindberg RLP, Kappos L, Leib S (2001) Matrix metalloproteinases: multifunctional effectors of inflammation in multiple sclerosis and bacterial meningitis. Brain Res Rev 36:249–257
de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, Kuiper J (1996) The influence of cytokines on the integrity of the blood– brain barrier in vitro. J Neuroimmunol 64:37–43
Nathan C (2002) Points of control in inflammation. Nature 420:846–852
Tracey KJ (2002) The inflammatory reflex. Nature 420:853–859
Banks WA (2005) Blood–brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des 11:973–984
Risau W, Wolburg H (1990) Development of the blood–brain barrier. Trends Neurosci 13:174–178
Huber JD, Hau VS, Borg L, Campos CR, Egleton RD, Davis TP (2002a) Blood–brain barrier tight junctions are altered during a 72-h exposure to lambda-carrageenan-induced inflammatory pain. Am J Physiol Heart Circ Physiol 283:H1531–H1537
Huber JD, Hau VS, Mark KS, Brown RC, Campos CR, Davis TP (2002b) Viability of microvascular endothelial cells to direct exposure of formalin, lambda-carrageenan, and complete Freund’s adjuvant. Eur J Pharmacol 450:297–304
de Simone R, Vissicchio F, Mingarelli C et al (2013) Branched-chain amino acids influence the immune properties of microglial cells and their responsiveness to pro-inflammatory signals. Biochim Biophys Acta 1832:650–659
Nagase H (1997) Activation mechanisms of matrix metalloproteinases. Biol Chem 378:151–160
Gu Z, Kaul M, Yan B et al (2002) S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science 297:1186–1190
Overall CM, Lopez-Otin C (2002) Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer 2:657–672
Pun PB, Lu J, Moochhala S (2009) Involvement of ROS in BBB dysfunction. Free Radic Res 43:348–364
Xaio H, Banks WA, Niehoff ML, Morley JE (2001) Effect of LPS on the permeability of the blood–brain barrier to insulin. Brain Res 896:36–42
Singh AK, Jiang Y (2004) How does peripheral lipopolysaccharide induce gene expression in the brain of rats? Toxicology 201:197–207
Bickel U, Grave B, Kang YS, del Rey A, Voigt K (1998) No increase in blood–brain barrier permeability after intraperitoneal injection of endotoxin in the rat. J Neuroimmunol 85:131–136
Acknowledgments
This research was supported by grants from the Programa de Pós-Graduação em Ciências da Saúde—Universidade do Extremo Sul Catarinense (UNESC), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Núcleo de Excelência em Neurociências Aplicadas de Santa Catarina (NENASC), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scaini, G., Morais, M.O.S., Galant, L.S. et al. Coadministration of Branched-Chain Amino Acids and Lipopolysaccharide Causes Matrix Metalloproteinase Activation and Blood–Brain Barrier Breakdown. Mol Neurobiol 50, 358–367 (2014). https://doi.org/10.1007/s12035-013-8618-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8618-0