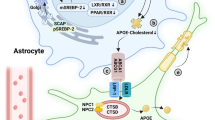
Abstract
Alzheimer’s disease (AD) is a complex multifactorial neurodegenerative disorder believed to be initiated by accumulation of amyloid β (Aβ)-related peptides derived from proteolytic processing of amyloid precursor protein (APP). Research over the past two decades provided a mechanistic link between cholesterol and AD pathogenesis. Genetic polymorphisms in genes regulating the pivotal points in cholesterol metabolism have been suggested to enhance the risk of developing AD. Altered neuronal membrane cholesterol level and/or subcellular distribution have been implicated in aberrant formation, aggregation, toxicity, and degradation of Aβ-related peptides. However, the results are somewhat contradictory and we still do not have a complete understanding on how cholesterol can influence AD pathogenesis. In this review, we summarize our current understanding on the role of cholesterol in regulating the production/function of Aβ-related peptides and also examine the therapeutic potential of regulating cholesterol homeostasis in the treatment of AD pathology.




Similar content being viewed by others
Abbreviations
- Aβ:
-
β-Amyloid
- ADAM:
-
A disintegrin and metalloproteinase domain containing protein
- ABC:
-
ATP-binding cassette
- ACAT:
-
Acyl-coenzyme-A cholesterol acyltransferase
- AD:
-
Alzheimer’s disease
- AICD:
-
APP intracellular C-terminal domain
- APH-1:
-
Anterior pharynx defective 1
- ApoE:
-
Apolipoprotein E
- APP:
-
Amyloid precursor protein
- BACE:
-
β-Site APP-cleaving enzyme
- BBB:
-
Blood–brain barrier
- CNS:
-
Central nervous system
- CTF:
-
C-terminal fragment
- IDE:
-
Insulin-degrading enzyme
- ER:
-
Endoplasmic reticulum
- GPI:
-
Glycosylphosphatidylinositol
- HDL:
-
High-density lipoprotein
- HMG-CoA:
-
3-Hydroxy-3-methylglutaryl-CoA
- HMGCR:
-
3-Hydroxy-3-methylglutaryl-CoA reductase
- LDL:
-
Low-density lipoprotein
- LDLR:
-
LDL receptor
- LRP:
-
LDL receptor-related protein
- LXR:
-
Liver X receptor
- NFTs:
-
Neurofibrillary tangles
- NPC:
-
Niemann–Pick type C
- PEN-2:
-
Presenilin enhancer 2
- PS1:
-
Presenilin 1
- PS2:
-
Presenilin 2
- SCAP:
-
SREBP cleavage-activating protein
- SREBP:
-
Sterol-regulated element-binding protein
- TACE:
-
Tumor necrosis factor-α-converting enzyme
References
Katzman R, Kawas C (1994) The epidemiology of dementia and Alzheimer disease. In: Terry RD, Katzman R, Bick KL (eds) Alzheimer disease. Raven, New York, pp 105–122
Katzov H, Chalmers K, Palmgren J, Andreasen N, Johansson B, Cairns NJ et al (2004) Genetic variants of ABCA1 modify Alzheimer disease risk and quantitative traits related to beta-amyloid metabolism. Hum Mutat 23(4):358–367
Whitehouse PJ (1997) Genesis of Alzheimer's disease. Neurology 48(5 Suppl 7):S2–S7
Bertram L, Tanzi RE (2005) The genetic epidemiology of neurodegenerative disease. J Clin Invest 115(6):1449–1457
Cummings JL (2004) Alzheimer's disease. N Engl J Med 351(1):56–67
NIA (2012) 2010 Alzheimer’s disease progress report: a deeper understanding. National Institute of Health. Available at http://www.nia.nih.gov/newsroom/announcements/2012/01/. Accessed 20 May 2012
Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE (2007) Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet 39(1):17–23
Holmes C (2002) Genotype and phenotype in Alzheimer's disease. Br J Psychiatry 180:131–134
St George-Hyslop PH, Petit A (2005) Molecular biology and genetics of Alzheimer's disease. C R Biol 328(2):119–130
Tanzi RE, Bertram L (2005) Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell 120(4):545–555
Coon KD, Myers AJ, Craig DW, Webster JA, Pearson JV, Lince DH et al (2007) A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer's disease. J Clin Psychiatry 68(4):613–618
Poirier J (2003) Apolipoprotein E and cholesterol metabolism in the pathogenesis and treatment of Alzheimer's disease. Trends Mol Med 9(3):94–101
Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS et al (1993) Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA 90(5):1977–1981
Poirier J, Baccichet A, Dea D, Gauthier S (1993) Cholesterol synthesis and lipoprotein reuptake during synaptic remodelling in hippocampus in adult rats. Neuroscience 55(1):81–90
Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML et al (2009) Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet 41(10):1088–1093
Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M et al (2009) Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet 41(10):1094–1099
Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, Boada M et al (2010) Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA 303(18):1832–1840
Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F et al (2007) The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet 39(2):168–177
Hollingworth P, Harold D, Jones L, Owen MJ, Williams J (2011) Alzheimer's disease genetics: current knowledge and future challenges. Int J Geriatr Psychiatry 26(8):793–802
Lambert JC, Amouyel P (2011) Genetics of Alzheimer's disease: new evidences for an old hypothesis? Curr Opin Genet Dev 21(3):295–301
Chen JH, Lin KP, Chen YC (2009) Risk factors for dementia. J Formos Med Assoc 108(10):754–764
Muller-Spahn F, Hock C (1999) Risk factors and differential diagnosis of Alzheimer's disease. Eur Arch Psychiatry Clin Neurosci 249(Suppl 3):37–42
Querfurth HW, LaFerla FM (2010) Alzheimer's disease. N Engl J Med 362(4):329–344
Brion JP, Anderton BH, Authelet M, Dayanandan R, Leroy K, Lovestone S et al (2001) Neurofibrillary tangles and tau phosphorylation. Biochem Soc Symp 67:81–88
Iqbal K, Alonso AC, Gong CX, Khatoon S, Pei JJ, Wang JZ et al (1998) Mechanisms of neurofibrillary degeneration and the formation of neurofibrillary tangles. J Neural Transm Suppl 53:169–180
Lee VM (1996) Regulation of tau phosphorylation in Alzheimer's disease. Ann N Y Acad Sci 777:107–113
Billingsley ML, Kincaid RL (1997) Regulated phosphorylation and dephosphorylation of tau protein: effects on microtubule interaction, intracellular trafficking and neurodegeneration. Biochem J 323(Pt 3):577–591
Johnson GV, Jenkins SM (1999) Tau protein in normal and Alzheimer's disease brain. J Alzheimers Dis 1(4–5):307–328
Bierer LM, Hof PR, Purohit DP, Carlin L, Schmeidler J, Davis KL et al (1995) Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer's disease. Arch Neurol 52(1):81–88
Lopez OL, DeKosky ST (2003) Neuropathology of Alzheimer's disease and mild cognitive impairment. Rev Neurol 37:155–163
Nelson PT, Braak H, Markesbery WR (2009) Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol 68(1):1–14
Selkoe DJ (2001) Alzheimer's disease: genes, proteins, and therapy. Physiol Rev 81(2):741–766
Clippingdale AB, Wade JD, Barrow CJ (2001) The amyloid-beta peptide and its role in Alzheimer's disease. J Pept Sci 7(5):227–249
Dickson DW (1997) The pathogenesis of senile plaques. J Neuropathol Exp Neurol 56(4):321–339
Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P et al (2000) Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA 283(12):1571–1577
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297(5580):353–356
Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC et al (2010) Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science 330(6012):1774
DeKosky ST, Scheff SW, Styren SD (1996) Structural correlates of cognition in dementia: quantification and assessment of synapse change. Neurodegeneration 5(4):417–421
Kar S, Slowikowski SP, Westaway D, Mount HT (2004) Interactions between beta-amyloid and central cholinergic neurons: implications for Alzheimer's disease. J Psychiatry Neurosci 29(6):427–441
Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW et al (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261(5123):921–923
Slooter AJ, Cruts M, Kalmijn S, Hofman A, Breteler MM, Van Broeckhoven C et al (1998) Risk estimates of dementia by apolipoprotein E genotypes from a population-based incidence study: the Rotterdam Study. Arch Neurol 55(7):964–968
Jarvik GP, Wijsman EM, Kukull WA, Schellenberg GD, Yu C, Larson EB (1995) Interactions of apolipoprotein E genotype, total cholesterol level, age, and sex in prediction of Alzheimer's disease: a case–control study. Neurology 45(6):1092–1096
Tomiyama T, Corder EH, Mori H (1999) Molecular pathogenesis of apolipoprotein E-mediated amyloidosis in late-onset Alzheimer's disease. Cell Mol Life Sci 56(3–4):268–279
Wisniewski T, Castano EM, Golabek A, Vogel T, Frangione B (1994) Acceleration of Alzheimer's fibril formation by apolipoprotein E in vitro. Am J Pathol 145(5):1030–1035
Carter DB, Dunn E, McKinley DD, Stratman NC, Boyle TP, Kuiper SL et al (2001) Human apolipoprotein E4 accelerates beta-amyloid deposition in APPsw transgenic mouse brain. Ann Neurol 50(4):468–475
Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ et al (2000) Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA 97(6):2892–2897
Sadowski M, Pankiewicz J, Scholtzova H, Ripellino JA, Li Y, Schmidt SD et al (2004) A synthetic peptide blocking the apolipoprotein E/beta-amyloid binding mitigates beta-amyloid toxicity and fibril formation in vitro and reduces beta-amyloid plaques in transgenic mice. Am J Pathol 165(3):937–948
Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R et al (2007) Transport pathways for clearance of human Alzheimer's amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab 27(5):909–918
Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB et al (2008) ApoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest 118(12):4002–4013
DeMattos RB, Cirrito JR, Parsadanian M, May PC, O'Dell MA, Taylor JW et al (2004) ApoE and clusterin cooperatively suppress Abeta levels and deposition: evidence that ApoE regulates extracellular Abeta metabolism in vivo. Neuron 41(2):193–202
Jiang Q, Lee CY, Mandrekar S, Wilkinson B, Cramer P, Zelcer N et al (2008) ApoE promotes the proteolytic degradation of Abeta. Neuron 58(5):681–693
Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J et al (2004) Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med 10(7):719–726
Kolsch H, Lutjohann D, Ludwig M, Schulte A, Ptok U, Jessen F et al (2002) Polymorphism in the cholesterol 24S-hydroxylase gene is associated with Alzheimer's disease. Mol Psychiatry 7(8):899–902
Borroni B, Archetti S, Agosti C, Akkawi N, Brambilla C, Caimi L et al (2004) Intronic CYP46 polymorphism along with ApoE genotype in sporadic Alzheimer disease: from risk factors to disease modulators. Neurobiol Aging 25(6):747–751
Combarros O, Infante J, Llorca J, Berciano J (2004) Genetic association of CYP46 and risk for Alzheimer's disease. Dement Geriatr Cogn Disord 18(3–4):257–260
Johansson A, Katzov H, Zetterberg H, Feuk L, Johansson B, Bogdanovic N et al (2004) Variants of CYP46A1 may interact with age and APOE to influence CSF Abeta42 levels in Alzheimer's disease. Hum Genet 114(6):581–587
Papassotiropoulos A, Streffer JR, Tsolaki M, Schmid S, Thal D, Nicosia F et al (2003) Increased brain beta-amyloid load, phosphorylated tau, and risk of Alzheimer disease associated with an intronic CYP46 polymorphism. Arch Neurol 60(1):29–35
Shibata N, Kawarai T, Lee JH, Lee HS, Shibata E, Sato C et al (2006) Association studies of cholesterol metabolism genes (CH25H, ABCA1 and CH24H) in Alzheimer's disease. Neurosci Lett 391(3):142–146
Wollmer MA, Streffer JR, Lutjohann D, Tsolaki M, Iakovidou V, Hegi T et al (2003) ABCA1 modulates CSF cholesterol levels and influences the age at onset of Alzheimer's disease. Neurobiol Aging 24(3):421–426
Di Paolo G, Kim TW (2011) Linking lipids to Alzheimer's disease: cholesterol and beyond. Nat Rev Neurosci 12(5):284–296
Matsuzaki T, Sasaki K, Hata J, Hirakawa Y, Fujimi K, Ninomiya T et al (2011) Association of Alzheimer disease pathology with abnormal lipid metabolism: the Hisayama Study. Neurology 77(11):1068–1075
Pappolla MA, Bryant-Thomas TK, Herbert D, Pacheco J, Fabra Garcia M, Manjon M et al (2003) Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Neurology 61(2):199–205
Reiss AB, Voloshyna I (2012) Regulation of cerebral cholesterol metabolism in Alzheimer disease. J Investig Med 60(3):576–582
Kuo YM, Emmerling MR, Bisgaier CL, Essenburg AD, Lampert HC, Drumm D et al (1998) Elevated low-density lipoprotein in Alzheimer's disease correlates with brain abeta 1–42 levels. Biochem Biophys Res Commun 252(3):711–715
Haag MD, Hofman A, Koudstaal PJ, Stricker BH, Breteler MM (2009) Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J Neurol Neurosurg Psychiatry 80(1):13–17
Sparks DL, Kryscio RJ, Sabbagh MN, Connor DJ, Sparks LM, Liebsack C (2008) Reduced risk of incident AD with elective statin use in a clinical trial cohort. Curr Alzheimer Res 5(4):416–421
Wolozin B, Wang SW, Li NC, Lee A, Lee TA, Kazis LE (2007) Simvastatin is associated with a reduced incidence of dementia and Parkinson's disease. BMC Med 5:20
Zamrini E, McGwin G, Roseman JM (2004) Association between statin use and Alzheimer's disease. Neuroepidemiology 23(1–2):94–98
Distl R, Meske V, Ohm TG (2001) Tangle-bearing neurons contain more free cholesterol than adjacent tangle-free neurons. Acta Neuropathol 101(6):547–554
Chan RB, Oliveira TG, Cortes EP, Honig LS, Duff KE, Small SA et al (2012) Comparative lipidomic analysis of mouse and human brain with Alzheimer disease. J Biol Chem 287(4):2678–2688
Martin M, Dotti CG, Ledesma MD (2010) Brain cholesterol in normal and pathological aging. Biochim Biophys Acta 1801(8):934–944
Bjorkhem I, Meaney S (2004) Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol 24(5):806–815
Dietschy JM, Turley SD (2004) Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res 45(8):1375–1397
Bu G (2009) Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat Rev Neurosci 10(5):333–344
Kim J, Basak JM, Holtzman DM (2009) The role of apolipoprotein E in Alzheimer's disease. Neuron 63(3):287–303
Shepardson NE, Shankar GM, Selkoe DJ (2011) Cholesterol level and statin use in Alzheimer disease: I. Review of epidemiological and preclinical studies. Arch Neurol 68(10):1239–1244
Verghese PB, Castellano JM, Holtzman DM (2011) Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol 10(3):241–252
Maron DJ, Fazio S, Linton MF (2000) Current perspectives on statins. Circulation 101(2):207–213
Martins IJ, Berger T, Sharman MJ, Verdile G, Fuller SJ, Martins RN (2009) Cholesterol metabolism and transport in the pathogenesis of Alzheimer's disease. J Neurochem 111(6):1275–1308
Serougne-Gautheron C, Chevallier F (1973) Time course of biosynthetic cholesterol in the adult rat brain. Biochim Biophys Acta 316(2):244–250
Bjorkhem I, Lutjohann D, Diczfalusy U, Stahle L, Ahlborg G, Wahren J (1998) Cholesterol homeostasis in human brain: turnover of 24S-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulation. J Lipid Res 39(8):1594–1600
Pfrieger FW (2003) Cholesterol homeostasis and function in neurons of the central nervous system. Cell Mol Life Sci 60(6):1158–1171
Ikonen E (2006) Mechanisms for cellular cholesterol transport: defects and human disease. Physiol Rev 86(4):1237–1261
Miller WL (1987) Structure of genes encoding steroidogenic enzymes. J Steroid Biochem 27(4–6):759–766
Goldstein JL, DeBose-Boyd RA, Brown MS (2006) Protein sensors for membrane sterols. Cell 124(1):35–46
Anderson RG (2003) Joe Goldstein and Mike Brown: from cholesterol homeostasis to new paradigms in membrane biology. Trends Cell Biol 13(10):534–539
Jurevics H, Morell P (1995) Cholesterol for synthesis of myelin is made locally, not imported into brain. J Neurochem 64(2):895–901
Beffert U, Danik M, Krzywkowski P, Ramassamy C, Berrada F, Poirier J (1998) The neurobiology of apolipoproteins and their receptors in the CNS and Alzheimer's disease. Brain Res Brain Res Rev 27(2):119–142
Karten B, Campenot RB, Vance DE, Vance JE (2006) Expression of ABCG1, but not ABCA1, correlates with cholesterol release by cerebellar astroglia. J Biol Chem 281(7):4049–4057
Wahrle SE, Jiang H, Parsadanian M, Legleiter J, Han X, Fryer JD et al (2004) ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J Biol Chem 279(39):40987–40993
Herz J (2009) Apolipoprotein E receptors in the nervous system. Curr Opin Lipidol 20(3):190–196
Puglielli L, Konopka G, Pack-Chung E, Ingano LA, Berezovska O, Hyman BT et al (2001) Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid beta-peptide. Nat Cell Biol 3(10):905–912
Rudel LL, Lee RG, Cockman TL (2001) Acyl coenzyme A: cholesterol acyltransferase types 1 and 2: structure and function in atherosclerosis. Curr Opin Lipidol 12(2):121–127
Bogdanovic N, Bretillon L, Lund EG, Diczfalusy U, Lannfelt L, Winblad B et al (2001) On the turnover of brain cholesterol in patients with Alzheimer's disease. Abnormal induction of the cholesterol-catabolic enzyme CYP46 in glial cells. Neurosci Lett 314(1–2):45–48
Lund EG, Guileyardo JM, Russell DW (1999) cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc Natl Acad Sci USA 96(13):7238–7243
Lund EG, Xie C, Kotti T, Turley SD, Dietschy JM, Russell DW (2003) Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J Biol Chem 278(25):22980–22988
Kim WS, Rahmanto AS, Kamili A, Rye KA, Guillemin GJ, Gelissen IC et al (2007) Role of ABCG1 and ABCA1 in regulation of neuronal cholesterol efflux to apolipoprotein E discs and suppression of amyloid-beta peptide generation. J Biol Chem 282(5):2851–2861
Hirsch-Reinshagen V, Maia LF, Burgess BL, Blain JF, Naus KE, McIsaac SA et al (2005) The absence of ABCA1 decreases soluble ApoE levels but does not diminish amyloid deposition in two murine models of Alzheimer disease. J Biol Chem 280(52):43243–43256
Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL et al (1997) Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem 272(6):3137–3140
Wang N, Lan D, Chen W, Matsuura F, Tall AR (2004) ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci USA 101(26):9774–9779
Wang N, Silver DL, Thiele C, Tall AR (2001) ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J Biol Chem 276(26):23742–23747
Wang Y, Rogers PM, Stayrook KR, Su C, Varga G, Shen Q et al (2008) The selective Alzheimer's disease indicator-1 gene (seladin-1/DHCR24) is a liver X receptor target gene. Mol Pharmacol 74(6):1716–1721
Sparks DL, Scheff SW, Hunsaker JC 3rd, Liu H, Landers T, Gross DR (1994) Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp Neurol 126(1):88–94
Schroeder F, Gallegos AM, Atshaves BP, Storey SM, McIntosh AL, Petrescu AD et al (2001) Recent advances in membrane microdomains: rafts, caveolae, and intracellular cholesterol trafficking. Exp Biol Med (Maywood) 226(10):873–890
Hayashi H, Igbavboa U, Hamanaka H, Kobayashi M, Fujita SC, Wood WG et al (2002) Cholesterol is increased in the exofacial leaflet of synaptic plasma membranes of human apolipoprotein E4 knock-in mice. Neuroreport 13(4):383–386
Wood WG, Schroeder F, Avdulov NA, Chochina SV, Igbavboa U (1999) Recent advances in brain cholesterol dynamics: transport, domains, and Alzheimer's disease. Lipids 34(3):225–234
Jacobson K, Mouritsen OG, Anderson RG (2007) Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol 9(1):7–14
Lingwood D, Simons K (2010) Lipid rafts as a membrane-organizing principle. Science 327(5961):46–50
Simons K, Ehehalt R (2002) Cholesterol, lipid rafts, and disease. J Clin Invest 110(5):597–603
Bouillot C, Prochiantz A, Rougon G, Allinquant B (1996) Axonal amyloid precursor protein expressed by neurons in vitro is present in a membrane fraction with caveolae-like properties. J Biol Chem 271(13):7640–7644
Lee SJ, Liyanage U, Bickel PE, Xia W, Lansbury PT Jr, Kosik KS (1998) A detergent-insoluble membrane compartment contains A beta in vivo. Nat Med 4(6):730–734
Vance JE (2006) Lipid imbalance in the neurological disorder, Niemann–Pick C disease. FEBS Lett 580(23):5518–5524
Vanier MT, Millat G (2003) Niemann–Pick disease type C. Clin Genet 64(4):269–281
Walkley SU, Suzuki K (2004) Consequences of NPC1 and NPC2 loss of function in mammalian neurons. Biochim Biophys Acta 1685(1–3):48–62
Jin LW, Shie FS, Maezawa I, Vincent I, Bird T (2004) Intracellular accumulation of amyloidogenic fragments of amyloid-beta precursor protein in neurons with Niemann–Pick type C defects is associated with endosomal abnormalities. Am J Pathol 164(3):975–985
Kodam A, Maulik M, Peake K, Amritraj A, Vetrivel KS, Thinakaran G et al (2010) Altered levels and distribution of amyloid precursor protein and its processing enzymes in Niemann–Pick type C1-deficient mouse brains. Glia 58(11):1267–1281
Runz H, Rietdorf J, Tomic I, de Bernard M, Beyreuther K, Pepperkok R et al (2002) Inhibition of intracellular cholesterol transport alters presenilin localization and amyloid precursor protein processing in neuronal cells. J Neurosci 22(5):1679–1689
Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH et al (1987) The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature 325(6106):733–736
O'Brien RJ, Wong PC (2011) Amyloid precursor protein processing and Alzheimer's disease. Annu Rev Neurosci 34:185–204
Price DL, Sisodia SS (1998) Mutant genes in familial Alzheimer's disease and transgenic models. Annu Rev Neurosci 21:479–505
Selkoe DJ (2008) Biochemistry and molecular biology of amyloid beta-protein and the mechanism of Alzheimer’s disease. Handb Clin Neurol 89:245–260
Small SA, Gandy S (2006) Sorting through the cell biology of Alzheimer's disease: intracellular pathways to pathogenesis. Neuron 52(1):15–31
Lichtenthaler SF (2012) Alpha-secretase cleavage of the amyloid precursor protein: proteolysis regulated by signaling pathways and protein trafficking. Curr Alzheimer Res 9(2):165–177
Sisodia SS (1992) Beta-amyloid precursor protein cleavage by a membrane-bound protease. Proc Natl Acad Sci USA 89(13):6075–6079
Thinakaran G, Koo EH (2008) Amyloid precursor protein trafficking, processing, and function. J Biol Chem 283(44):29615–29619
Allinson TM, Parkin ET, Turner AJ, Hooper NM (2003) ADAMs family members as amyloid precursor protein alpha-secretases. J Neurosci Res 74(3):342–352
Kojro E, Fahrenholz F (2005) The non-amyloidogenic pathway: structure and function of alpha-secretases. Subcell Biochem 38:105–127
Esch FS, Keim PS, Beattie EC, Blacher RW, Culwell AR, Oltersdorf T et al (1990) Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science 248(4959):1122–1124
Grziwa B, Grimm MO, Masters CL, Beyreuther K, Hartmann T, Lichtenthaler SF (2003) The transmembrane domain of the amyloid precursor protein in microsomal membranes is on both sides shorter than predicted. J Biol Chem 278(9):6803–6808
Qi-Takahara Y, Morishima-Kawashima M, Tanimura Y, Dolios G, Hirotani N, Horikoshi Y et al (2005) Longer forms of amyloid beta protein: implications for the mechanism of intramembrane cleavage by gamma-secretase. J Neurosci 25(2):436–445
Selkoe DJ, Wolfe MS (2007) Presenilin: running with scissors in the membrane. Cell 131(2):215–221
Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P et al (1999) Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286(5440):735–741
Chavez-Gutierrez L, Bammens L, Benilova I, Vandersteen A, Benurwar M, Borgers M et al (2012) The mechanism of gamma-secretase dysfunction in familial Alzheimer disease. EMBO J 31(10):2261–2274
Takami M, Nagashima Y, Sano Y, Ishihara S, Morishima-Kawashima M, Funamoto S et al (2009) Gamma-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci 29(41):13042–13052
Cook DG, Forman MS, Sung JC, Leight S, Kolson DL, Iwatsubo T et al (1997) Alzheimer's A beta(1-42) is generated in the endoplasmic reticulum/intermediate compartment of NT2N cells. Nat Med 3(9):1021–1023
Greenfield JP, Tsai J, Gouras GK, Hai B, Thinakaran G, Checler F et al (1999) Endoplasmic reticulum and trans-Golgi network generate distinct populations of Alzheimer beta-amyloid peptides. Proc Natl Acad Sci USA 96(2):742–747
Koo EH, Squazzo SL (1994) Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem 269(26):17386–17389
LaFerla FM, Green KN, Oddo S (2007) Intracellular amyloid-beta in Alzheimer's disease. Nat Rev Neurosci 8(7):499–509
Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H et al (2002) Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol 161(5):1869–1879
Cole SL, Vassar R (2007) The Alzheimer's disease beta-secretase enzyme, BACE1. Mol Neurodegener 2:22
Francis R, McGrath G, Zhang J, Ruddy DA, Sym M, Apfeld J et al (2002) Aph-1 and pen-2 are required for Notch pathway signaling, gamma-secretase cleavage of betaAPP, and presenilin protein accumulation. Dev Cell 3(1):85–97
Levitan D, Lee J, Song L, Manning R, Wong G, Parker E et al (2001) PS1 N- and C-terminal fragments form a complex that functions in APP processing and Notch signaling. Proc Natl Acad Sci USA 98(21):12186–12190
Serneels L, Van Biervliet J, Craessaerts K, Dejaegere T, Horre K, Van Houtvin T et al (2009) Gamma-Secretase heterogeneity in the Aph1 subunit: relevance for Alzheimer's disease. Science 324(5927):639–642
Steiner H, Fluhrer R, Haass C (2008) Intramembrane proteolysis by gamma-secretase. J Biol Chem 283(44):29627–29631
Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ (1999) Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature 398(6727):513–517
Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A et al (2000) Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and betaAPP processing. Nature 407(6800):48–54
Iwatsubo T (2004) Assembly and activation of the gamma-secretase complex: roles of presenilin cofactors. Mol Psychiatry 9(1):8–10
De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS et al (1999) A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature 398(6727):518–522
Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM et al (2010) Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 141(7):1146–1158
Zhang C, Wu B, Beglopoulos V, Wines-Samuelson M, Zhang D, Dragatsis I et al (2009) Presenilins are essential for regulating neurotransmitter release. Nature 460(7255):632–636
Mills J, Reiner PB (1999) Regulation of amyloid precursor protein cleavage. J Neurochem 72(2):443–460
Roberson MR, Harrell LE (1997) Cholinergic activity and amyloid precursor protein metabolism. Brain Res Brain Res Rev 25(1):50–69
Bodovitz S, Klein WL (1996) Cholesterol modulates alpha-secretase cleavage of amyloid precursor protein. J Biol Chem 271(8):4436–4440
Frears ER, Stephens DJ, Walters CE, Davies H, Austen BM (1999) The role of cholesterol in the biosynthesis of beta-amyloid. Neuroreport 10(8):1699–1705
Abad-Rodriguez J, Ledesma MD, Craessaerts K, Perga S, Medina M, Delacourte A et al (2004) Neuronal membrane cholesterol loss enhances amyloid peptide generation. J Cell Biol 167(5):953–960
Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P et al (2001) Simvastatin strongly reduces levels of Alzheimer's disease beta-amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci USA 98(10):5856–5861
Kojro E, Gimpl G, Lammich S, Marz W, Fahrenholz F (2001) Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha-secretase ADAM 10. Proc Natl Acad Sci USA 98(10):5815–5820
Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K (1998) Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc Natl Acad Sci USA 95(11):6460–6464
Kalvodova L, Kahya N, Schwille P, Ehehalt R, Verkade P, Drechsel D et al (2005) Lipids as modulators of proteolytic activity of BACE: involvement of cholesterol, glycosphingolipids, and anionic phospholipids in vitro. J Biol Chem 280(44):36815–36823
Osenkowski P, Ye W, Wang R, Wolfe MS, Selkoe DJ (2008) Direct and potent regulation of gamma-secretase by its lipid microenvironment. J Biol Chem 283(33):22529–22540
Vetrivel KS, Cheng H, Lin W, Sakurai T, Li T, Nukina N et al (2004) Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J Biol Chem 279(43):44945–44954
Wahrle S, Das P, Nyborg AC, McLendon C, Shoji M, Kawarabayashi T et al (2002) Cholesterol-dependent gamma-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol Dis 9(1):11–23
Ehehalt R, Keller P, Haass C, Thiele C, Simons K (2003) Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol 160(1):113–123
Parkin ET, Turner AJ, Hooper NM (1999) Amyloid precursor protein, although partially detergent-insoluble in mouse cerebral cortex, behaves as an atypical lipid raft protein. Biochem J 344(Pt 1):23–30
Marquer C, Devauges V, Cossec JC, Liot G, Lecart S, Saudou F et al (2011) Local cholesterol increase triggers amyloid precursor protein-Bace1 clustering in lipid rafts and rapid endocytosis. FASEB J 25(4):1295–1305
Cordy JM, Hussain I, Dingwall C, Hooper NM, Turner AJ (2003) Exclusively targeting beta-secretase to lipid rafts by GPI-anchor addition up-regulates beta-site processing of the amyloid precursor protein. Proc Natl Acad Sci USA 100(20):11735–11740
Vetrivel KS, Barman A, Chen Y, Nguyen PD, Wagner SL, Prabhakar R et al (2011) Loss of cleavage at β'-site contributes to apparent increase in β-amyloid peptide (Aβ) secretion by β-secretase (BACE1)-glycosylphosphatidylinositol (GPI) processing of amyloid precursor protein. J Biol Chem 286(29):26166–26177
Cole SL, Grudzien A, Manhart IO, Kelly BL, Oakley H, Vassar R (2005) Statins cause intracellular accumulation of amyloid precursor protein, beta-secretase-cleaved fragments, and amyloid beta-peptide via an isoprenoid-dependent mechanism. J Biol Chem 280(19):18755–18770
Hansen GH, Niels-Christiansen LL, Thorsen E, Immerdal L, Danielsen EM (2000) Cholesterol depletion of enterocytes. Effect on the Golgi complex and apical membrane trafficking. J Biol Chem 275(7):5136–5142
Hao M, Mukherjee S, Sun Y, Maxfield FR (2004) Effects of cholesterol depletion and increased lipid unsaturation on the properties of endocytic membranes. J Biol Chem 279(14):14171–14178
Vetrivel KS, Thinakaran G (2010) Membrane rafts in Alzheimer's disease beta-amyloid production. Biochim Biophys Acta 1801(8):860–867
Davis W Jr (2008) The cholesterol transport inhibitor U18666a regulates amyloid precursor protein metabolism and trafficking in N2aAPP "Swedish" cells. Curr Alzheimer Res 5(5):448–456
Huttunen HJ, Puglielli L, Ellis BC, MacKenzie Ingano LA, Kovacs DM (2009) Novel N-terminal cleavage of APP precludes Abeta generation in ACAT-defective AC29 cells. J Mol Neurosci 37(1):6–15
Koldamova RP, Lefterov IM, Staufenbiel M, Wolfe D, Huang S, Glorioso JC et al (2005) The liver X receptor ligand T0901317 decreases amyloid beta production in vitro and in a mouse model of Alzheimer's disease. J Biol Chem 280(6):4079–4088
Sun Y, Yao J, Kim TW, Tall AR (2003) Expression of liver X receptor target genes decreases cellular amyloid beta peptide secretion. J Biol Chem 278(30):27688–27694
Sawamura N, Ko M, Yu W, Zou K, Hanada K, Suzuki T et al (2004) Modulation of amyloid precursor protein cleavage by cellular sphingolipids. J Biol Chem 279(12):11984–11991
Beel AJ, Sakakura M, Barrett PJ, Sanders CR (2010) Direct binding of cholesterol to the amyloid precursor protein: an important interaction in lipid-Alzheimer's disease relationships? Biochim Biophys Acta 1801(8):975–982
Yao ZX, Papadopoulos V (2002) Function of beta-amyloid in cholesterol transport: a lead to neurotoxicity. FASEB J 16(12):1677–1679
Esler WP, Stimson ER, Ghilardi JR, Lu YA, Felix AM, Vinters HV et al (1996) Point substitution in the central hydrophobic cluster of a human beta-amyloid congener disrupts peptide folding and abolishes plaque competence. Biochemistry 35(44):13914–13921
Massi F, Straub JE (2001) Probing the origins of increased activity of the E22Q "Dutch" mutant Alzheimer's beta-amyloid peptide. Biophys J 81(2):697–709
Schmechel D, Sullivan P, Mace B, Sawyer J, Rudel L (2002) High saturated fat diets are associated with abeta deposition in primates. Neurobiol Aging 23(1):S323
Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS et al (2000) Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol Dis 7(4):321–331
Refolo LM, Pappolla MA, LaFrancois J, Malester B, Schmidt SD, Thomas-Bryant T et al (2001) A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer's disease. Neurobiol Dis 8(5):890–899
Ghribi O, Larsen B, Schrag M, Herman MM (2006) High cholesterol content in neurons increases BACE, beta-amyloid, and phosphorylated tau levels in rabbit hippocampus. Exp Neurol 200(2):460–467
Hooijmans CR, Rutters F, Dederen PJ, Gambarota G, Veltien A, van Groen T et al (2007) Changes in cerebral blood volume and amyloid pathology in aged Alzheimer APP/PS1 mice on a docosahexaenoic acid (DHA) diet or cholesterol enriched Typical Western Diet (TWD). Neurobiol Dis 28(1):16–29
Hooijmans CR, Van der Zee CE, Dederen PJ, Brouwer KM, Reijmer YD, van Groen T et al (2009) DHA and cholesterol containing diets influence Alzheimer-like pathology, cognition and cerebral vasculature in APPswe/PS1dE9 mice. Neurobiol Dis 33(3):482–498
Jaya Prasanthi RP, Schommer E, Thomasson S, Thompson A, Feist G, Ghribi O (2008) Regulation of beta-amyloid levels in the brain of cholesterol-fed rabbit, a model system for sporadic Alzheimer's disease. Mech Ageing Dev 129(11):649–655
Levin-Allerhand JA, Lominska CE, Smith JD (2002) Increased amyloid- levels in APPSWE transgenic mice treated chronically with a physiological high-fat high-cholesterol diet. J Nutr Health Aging 6(5):315–319
Li L, Cao D, Garber DW, Kim H, Fukuchi K (2003) Association of aortic atherosclerosis with cerebral beta-amyloidosis and learning deficits in a mouse model of Alzheimer's disease. Am J Pathol 163(6):2155–2164
Pedrini S, Thomas C, Brautigam H, Schmeidler J, Ho L, Fraser P et al (2009) Dietary composition modulates brain mass and solubilizable Abeta levels in a mouse model of aggressive Alzheimer's amyloid pathology. Mol Neurodegener 4:40
Shie FS, Jin LW, Cook DG, Leverenz JB, LeBoeuf RC (2002) Diet-induced hypercholesterolemia enhances brain A beta accumulation in transgenic mice. Neuroreport 13(4):455–459
Thirumangalakudi L, Prakasam A, Zhang R, Bimonte-Nelson H, Sambamurti K, Kindy MS et al (2008) High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J Neurochem 106(1):475–485
Cibickova L, Hyspler R, Micuda S, Cibicek N, Zivna H, Jun D et al (2009) The influence of simvastatin, atorvastatin and high-cholesterol diet on acetylcholinesterase activity, amyloid beta and cholesterol synthesis in rat brain. Steroids 74(1):13–19
George AJ, Holsinger RM, McLean CA, Laughton KM, Beyreuther K, Evin G et al (2004) APP intracellular domain is increased and soluble Abeta is reduced with diet-induced hypercholesterolemia in a transgenic mouse model of Alzheimer disease. Neurobiol Dis 16(1):124–132
Howland DS, Trusko SP, Savage MJ, Reaume AG, Lang DM, Hirsch JD et al (1998) Modulation of secreted beta-amyloid precursor protein and amyloid beta-peptide in brain by cholesterol. J Biol Chem 273(26):16576–16582
Petanceska SS, DeRosa S, Olm V, Diaz N, Sharma A, Thomas-Bryant T et al (2002) Statin therapy for Alzheimer's disease: will it work? J Mol Neurosci 19(1–2):155–161
Park IH, Hwang EM, Hong HS, Boo JH, Oh SS, Lee J et al (2003) Lovastatin enhances Abeta production and senile plaque deposition in female Tg2576 mice. Neurobiol Aging 24(5):637–643
Bales KR, Verina T, Cummins DJ, Du Y, Dodel RC, Saura J et al (1999) Apolipoprotein E is essential for amyloid deposition in the APP(V717F) transgenic mouse model of Alzheimer's disease. Proc Natl Acad Sci USA 96(26):15233–15238
Bales KR, Verina T, Dodel RC, Du Y, Altstiel L, Bender M et al (1997) Lack of apolipoprotein E dramatically reduces amyloid beta-peptide deposition. Nat Genet 17(3):263–264
Holtzman DM, Fagan AM, Mackey B, Tenkova T, Sartorius L, Paul SM et al (2000) Apolipoprotein E facilitates neuritic and cerebrovascular plaque formation in an Alzheimer's disease model. Ann Neurol 47(6):739–747
Irizarry MC, Cheung BS, Rebeck GW, Paul SM, Bales KR, Hyman BT (2000) Apolipoprotein E affects the amount, form, and anatomical distribution of amyloid beta-peptide deposition in homozygous APP(V717F) transgenic mice. Acta Neuropathol 100(5):451–458
Cao D, Fukuchi K, Wan H, Kim H, Li L (2006) Lack of LDL receptor aggravates learning deficits and amyloid deposits in Alzheimer transgenic mice. Neurobiol Aging 27(11):1632–1643
Fryer JD, Demattos RB, McCormick LM, O'Dell MA, Spinner ML, Bales KR et al (2005) The low density lipoprotein receptor regulates the level of central nervous system human and murine apolipoprotein E but does not modify amyloid plaque pathology in PDAPP mice. J Biol Chem 280(27):25754–25759
Kim J, Castellano JM, Jiang H, Basak JM, Parsadanian M, Pham V et al (2009) Overexpression of low-density lipoprotein receptor in the brain markedly inhibits amyloid deposition and increases extracellular A beta clearance. Neuron 64(5):632–644
Fagan AM, Christopher E, Taylor JW, Parsadanian M, Spinner M, Watson M et al (2004) ApoAI deficiency results in marked reductions in plasma cholesterol but no alterations in amyloid-beta pathology in a mouse model of Alzheimer's disease-like cerebral amyloidosis. Am J Pathol 165(4):1413–1422
Crameri A, Biondi E, Kuehnle K, Lutjohann D, Thelen KM, Perga S et al (2006) The role of seladin-1/DHCR24 in cholesterol biosynthesis, APP processing and Abeta generation in vivo. EMBO J 25(2):432–443
Greeve I, Hermans-Borgmeyer I, Brellinger C, Kasper D, Gomez-Isla T, Behl C et al (2000) The human DIMINUTO/DWARF1 homolog seladin-1 confers resistance to Alzheimer's disease-associated neurodegeneration and oxidative stress. J Neurosci 20(19):7345–7352
Iivonen S, Hiltunen M, Alafuzoff I, Mannermaa A, Kerokoski P, Puolivali J et al (2002) Seladin-1 transcription is linked to neuronal degeneration in Alzheimer's disease. Neuroscience 113(2):301–310
Ledesma MD, Abad-Rodriguez J, Galvan C, Biondi E, Navarro P, Delacourte A et al (2003) Raft disorganization leads to reduced plasmin activity in Alzheimer's disease brains. EMBO Rep 4(12):1190–1196
Halford RW, Russell DW (2009) Reduction of cholesterol synthesis in the mouse brain does not affect amyloid formation in Alzheimer's disease, but does extend lifespan. Proc Natl Acad Sci USA 106(9):3502–3506
Koldamova R, Staufenbiel M, Lefterov I (2005) Lack of ABCA1 considerably decreases brain ApoE level and increases amyloid deposition in APP23 mice. J Biol Chem 280(52):43224–43235
Wahrle SE, Jiang H, Parsadanian M, Hartman RE, Bales KR, Paul SM et al (2005) Deletion of Abca1 increases Abeta deposition in the PDAPP transgenic mouse model of Alzheimer disease. J Biol Chem 280(52):43236–43242
Wahrle SE, Jiang H, Parsadanian M, Kim J, Li A, Knoten A et al (2008) Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J Clin Invest 118(2):671–682
Bryleva EY, Rogers MA, Chang CC, Buen F, Harris BT, Rousselet E et al (2010) ACAT1 gene ablation increases 24(S)-hydroxycholesterol content in the brain and ameliorates amyloid pathology in mice with AD. Proc Natl Acad Sci USA 107(7):3081–3086
Hutter-Paier B, Huttunen HJ, Puglielli L, Eckman CB, Kim DY, Hofmeister A et al (2004) The ACAT inhibitor CP-113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer's disease. Neuron 44(2):227–238
Michaki V, Guix FX, Vennekens K, Munck S, Dingwall C, Davis JB et al (2012) Down-regulation of the ATP-binding cassette transporter 2 (Abca2) reduces amyloid-beta production by altering nicastrin maturation and intracellular localization. J Biol Chem 287(2):1100–1111
Yamazaki T, Chang TY, Haass C, Ihara Y (2001) Accumulation and aggregation of amyloid beta-protein in late endosomes of Niemann–Pick type C cells. J Biol Chem 276(6):4454–4460
Annaert WG, Levesque L, Craessaerts K, Dierinck I, Snellings G, Westaway D et al (1999) Presenilin 1 controls gamma-secretase processing of amyloid precursor protein in pre-Golgi compartments of hippocampal neurons. J Cell Biol 147(2):277–294
Burns M, Gaynor K, Olm V, Mercken M, LaFrancois J, Wang L et al (2003) Presenilin redistribution associated with aberrant cholesterol transport enhances beta-amyloid production in vivo. J Neurosci 23(13):5645–5649
Borbon IA, Erickson RP (2011) Interactions of Npc1 and amyloid accumulation/deposition in the APP/PS1 mouse model of Alzheimer's. J Appl Genet 52(2):213–218
Maulik M, Ghoshal B, Kim J, Wang Y, Yang J, Westaway D et al. (2012) Mutant human APP exacerbates pathology in a mouse model of NPC and its reversal by a beta-cyclodextrin. Hum Mol Genet (in press)
Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL et al (1992) Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature 359(6393):322–325
Shoji M (2002) Cerebrospinal fluid Abeta40 and Abeta42: natural course and clinical usefulness. Front Biosci 7:d997–1006
Vigo-Pelfrey C, Lee D, Keim P, Lieberburg I, Schenk DB (1993) Characterization of beta-amyloid peptide from human cerebrospinal fluid. J Neurochem 61(5):1965–1968
Giuffrida ML, Caraci F, Pignataro B, Cataldo S, De Bona P, Bruno V et al (2009) Beta-amyloid monomers are neuroprotective. J Neurosci 29(34):10582–10587
Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ et al (2005) Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci 8(1):79–84
Dahlgren KN, Manelli AM, Stine WB Jr, Baker LK, Krafft GA, LaDu MJ (2002) Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem 277(35):32046–32053
Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol 8(2):101–112
Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW et al (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300(5618):486–489
Lesne S, Kotilinek L, Ashe KH (2008) Plaque-bearing mice with reduced levels of oligomeric amyloid-beta assemblies have intact memory function. Neuroscience 151(3):745–749
Roychaudhuri R, Yang M, Hoshi MM, Teplow DB (2009) Amyloid beta-protein assembly and Alzheimer disease. J Biol Chem 284(8):4749–4753
Shankar GM, Walsh DM (2009) Alzheimer's disease: synaptic dysfunction and Abeta. Mol Neurodegener 4:48
Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L et al (1999) Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol 155(3):853–862
McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K et al (1999) Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol 46(6):860–866
Tomic JL, Pensalfini A, Head E, Glabe CG (2009) Soluble fibrillar oligomer levels are elevated in Alzheimer's disease brain and correlate with cognitive dysfunction. Neurobiol Dis 35(3):352–358
Schneider A, Schulz-Schaeffer W, Hartmann T, Schulz JB, Simons M (2006) Cholesterol depletion reduces aggregation of amyloid-beta peptide in hippocampal neurons. Neurobiol Dis 23(3):573–577
Rushworth JV, Hooper NM (2010) Lipid rafts: linking Alzheimer's amyloid-beta production, aggregation, and toxicity at neuronal membranes. Int J Alzheimers Dis 2011:603052
Kakio A, Nishimoto S, Yanagisawa K, Kozutsumi Y, Matsuzaki K (2002) Interactions of amyloid beta-protein with various gangliosides in raft-like membranes: importance of GM1 ganglioside-bound form as an endogenous seed for Alzheimer amyloid. Biochemistry 41(23):7385–7390
Matsuzaki K, Kato K, Yanagisawa K (2010) Abeta polymerization through interaction with membrane gangliosides. Biochim Biophys Acta 1801(8):868–877
McLaurin J, Franklin T, Fraser PE, Chakrabartty A (1998) Structural transitions associated with the interaction of Alzheimer beta-amyloid peptides with gangliosides. J Biol Chem 273(8):4506–4515
Choo-Smith LP, Garzon-Rodriguez W, Glabe CG, Surewicz WK (1997) Acceleration of amyloid fibril formation by specific binding of Abeta-(1–40) peptide to ganglioside-containing membrane vesicles. J Biol Chem 272(37):22987–22990
Kim SI, Yi JS, Ko YG (2006) Amyloid beta oligomerization is induced by brain lipid rafts. J Cell Biochem 99(3):878–889
Okada T, Ikeda K, Wakabayashi M, Ogawa M, Matsuzaki K (2008) Formation of toxic Abeta(1–40) fibrils on GM1 ganglioside-containing membranes mimicking lipid rafts: polymorphisms in Abeta(1–40) fibrils. J Mol Biol 382(4):1066–1074
Zampagni M, Evangelisti E, Cascella R, Liguri G, Becatti M, Pensalfini A et al (2010) Lipid rafts are primary mediators of amyloid oxidative attack on plasma membrane. J Mol Med 88(6):597–608
Yahi N, Aulas A, Fantini J (2010) How cholesterol constrains glycolipid conformation for optimal recognition of Alzheimer's beta amyloid peptide (Abeta1–40). PLoS One 5(2):e9079
Lin MS, Chen LY, Wang SS, Chang Y, Chen WY (2008) Examining the levels of ganglioside and cholesterol in cell membrane on attenuation the cytotoxicity of beta-amyloid peptide. Colloids Surf B Biointerfaces 65(2):172–177
Abramov AY, Ionov M, Pavlov E, Duchen MR (2011) Membrane cholesterol content plays a key role in the neurotoxicity of beta-amyloid: implications for Alzheimer's disease. Aging Cell 10(4):595–603
Ferrera P, Mercado-Gomez O, Silva-Aguilar M, Valverde M, Arias C (2008) Cholesterol potentiates beta-amyloid-induced toxicity in human neuroblastoma cells: involvement of oxidative stress. Neurochem Res 33(8):1509–1517
Wang SS, Rymer DL, Good TA (2001) Reduction in cholesterol and sialic acid content protects cells from the toxic effects of beta-amyloid peptides. J Biol Chem 276(45):42027–42034
Nicholson AM, Ferreira A (2009) Increased membrane cholesterol might render mature hippocampal neurons more susceptible to beta-amyloid-induced calpain activation and tau toxicity. J Neurosci 29(14):4640–4651
Fernandez A, Llacuna L, Fernandez-Checa JC, Colell A (2009) Mitochondrial cholesterol loading exacerbates amyloid beta peptide-induced inflammation and neurotoxicity. J Neurosci 29(20):6394–6405
Gonzalo-Ruiz A, Perez JL, Sanz JM, Geula C, Arevalo J (2006) Effects of lipids and aging on the neurotoxicity and neuronal loss caused by intracerebral injections of the amyloid-beta peptide in the rat. Exp Neurol 197(1):41–55
Cecchi C, Rosati F, Pensalfini A, Formigli L, Nosi D, Liguri G et al (2008) Seladin-1/DHCR24 protects neuroblastoma cells against Abeta toxicity by increasing membrane cholesterol content. J Cell Mol Med 12(5B):1990–2002
Arispe N, Doh M (2002) Plasma membrane cholesterol controls the cytotoxicity of Alzheimer's disease AbetaP (1–40) and (1–42) peptides. FASEB J 16(12):1526–1536
Sponne I, Fifre A, Koziel V, Oster T, Olivier JL, Pillot T (2004) Membrane cholesterol interferes with neuronal apoptosis induced by soluble oligomers but not fibrils of amyloid-beta peptide. FASEB J 18(7):836–838
Yip CM, Elton EA, Darabie AA, Morrison MR, McLaurin J (2001) Cholesterol, a modulator of membrane-associated Abeta-fibrillogenesis and neurotoxicity. J Mol Biol 311(4):723–734
Zhou Y, Richardson JS (1996) Cholesterol protects PC12 cells from beta-amyloid induced calcium disordering and cytotoxicity. Neuroreport 7(15–17):2487–2490
Allaman I, Gavillet M, Belanger M, Laroche T, Viertl D, Lashuel HA et al (2010) Amyloid-beta aggregates cause alterations of astrocytic metabolic phenotype: impact on neuronal viability. J Neurosci 30(9):3326–3338
Chung H, Brazil MI, Soe TT, Maxfield FR (1999) Uptake, degradation, and release of fibrillar and soluble forms of Alzheimer's amyloid beta-peptide by microglial cells. J Biol Chem 274(45):32301–32308
D'Andrea MR, Nagele RG, Wang HY, Peterson PA, Lee DH (2001) Evidence that neurones accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer's disease. Histopathology 38(2):120–134
Ditaranto K, Tekirian TL, Yang AJ (2001) Lysosomal membrane damage in soluble Abeta-mediated cell death in Alzheimer's disease. Neurobiol Dis 8(1):19–31
Mohamed A, Posse de Chaves E (2011) Abeta internalization by neurons and glia. Int J Alzheimers Dis 2011:127984
Pihlaja R, Koistinaho J, Malm T, Sikkila H, Vainio S, Koistinaho M (2008) Transplanted astrocytes internalize deposited beta-amyloid peptides in a transgenic mouse model of Alzheimer's disease. Glia 56(2):154–163
Song MS, Baker GB, Todd KG, Kar S (2011) Inhibition of beta-amyloid1–42 internalization attenuates neuronal death by stabilizing the endosomal-lysosomal system in rat cortical cultured neurons. Neuroscience 178:181–188
Wyss-Coray T, Lin C, Yan F, Yu GQ, Rohde M, McConlogue L et al (2001) TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat Med 7(5):612–618
Lai AY, McLaurin J (2011) Mechanisms of amyloid-beta peptide uptake by neurons: the role of lipid rafts and lipid raft-associated proteins. Int J Alzheimers Dis 2011:548380
Chafekar SM, Baas F, Scheper W (2008) Oligomer-specific Abeta toxicity in cell models is mediated by selective uptake. Biochim Biophys Acta 1782(9):523–531
Mandrekar S, Jiang Q, Lee CY, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE (2009) Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. J Neurosci 29(13):4252–4262
Yu C, Nwabuisi-Heath E, Laxton K, Ladu MJ (2010) Endocytic pathways mediating oligomeric Abeta42 neurotoxicity. Mol Neurodegener 5:19
Saavedra L, Mohamed A, Ma V, Kar S, de Chaves EP (2007) Internalization of beta-amyloid peptide by primary neurons in the absence of apolipoprotein E. J Biol Chem 282(49):35722–35732
Singh TD, Park SY, Bae JS, Yun Y, Bae YC, Park RW et al (2010) MEGF10 functions as a receptor for the uptake of amyloid-beta. FEBS Lett 584(18):3936–3942
Patel AN, Jhamandas JH (2012) Neuronal receptors as targets for the action of amyloid-beta protein (Abeta) in the brain. Expert Rev Mol Med 14:e2
Eckman EA, Eckman CB (2005) Abeta-degrading enzymes: modulators of Alzheimer's disease pathogenesis and targets for therapeutic intervention. Biochem Soc Trans 33(Pt 5):1101–1105
Miners JS, Baig S, Palmer J, Palmer LE, Kehoe PG, Love S (2008) Abeta-degrading enzymes in Alzheimer's disease. Brain Pathol 18(2):240–252
Fan J, Donkin J, Wellington C (2009) Greasing the wheels of Abeta clearance in Alzheimer's disease: the role of lipids and apolipoprotein E. Biofactors 35(3):239–248
Vekrellis K, Ye Z, Qiu WQ, Walsh D, Hartley D, Chesneau V et al (2000) Neurons regulate extracellular levels of amyloid beta-protein via proteolysis by insulin-degrading enzyme. J Neurosci 20(5):1657–1665
Bulloj A, Leal MC, Surace EI, Zhang X, Xu H, Ledesma MD et al (2008) Detergent resistant membrane-associated IDE in brain tissue and cultured cells: relevance to Abeta and insulin degradation. Mol Neurodegener 3:22
Kanemitsu H, Tomiyama T, Mori H (2003) Human neprilysin is capable of degrading amyloid beta peptide not only in the monomeric form but also the pathological oligomeric form. Neurosci Lett 350(2):113–116
Sato K, Tanabe C, Yonemura Y, Watahiki H, Zhao Y, Yagishita S et al (2012) Localization of mature neprilysin in lipid rafts. J Neurosci Res 90(4):870–877
Hama E, Shirotani K, Iwata N, Saido TC (2004) Effects of neprilysin chimeric proteins targeted to subcellular compartments on amyloid beta peptide clearance in primary neurons. J Biol Chem 279(29):30259–30264
Stefani M, Liguri G (2009) Cholesterol in Alzheimer's disease: unresolved questions. Curr Alzheimer Res 6(1):15–29
Lee CY, Tse W, Smith JD, Landreth GE (2012) Apolipoprotein E promotes beta-amyloid trafficking and degradation by modulating microglial cholesterol levels. J Biol Chem 287(3):2032–2044
Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA (2000) Statins and the risk of dementia. Lancet 356(9242):1627–1631
Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G (2000) Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol 57(10):1439–1443
Rockwood K, Kirkland S, Hogan DB, MacKnight C, Merry H, Verreault R et al (2002) Use of lipid-lowering agents, indication bias, and the risk of dementia in community-dwelling elderly people. Arch Neurol 59(2):223–227
Yaffe K, Barrett-Connor E, Lin F, Grady D (2002) Serum lipoprotein levels, statin use, and cognitive function in older women. Arch Neurol 59(3):378–384
Friedhoff LT, Cullen EI, Geoghagen NS, Buxbaum JD (2001) Treatment with controlled-release lovastatin decreases serum concentrations of human beta-amyloid (A beta) peptide. Int J Neuropsychopharmacol 4(2):127–130
Vega GL, Weiner MF, Lipton AM, Von Bergmann K, Lutjohann D, Moore C et al (2003) Reduction in levels of 24S-hydroxycholesterol by statin treatment in patients with Alzheimer disease. Arch Neurol 60(4):510–515
Ostrowski SM, Wilkinson BL, Golde TE, Landreth G (2007) Statins reduce amyloid-beta production through inhibition of protein isoprenylation. J Biol Chem 282(37):26832–26844
Fonseca AC, Resende R, Oliveira CR, Pereira CM (2010) Cholesterol and statins in Alzheimer's disease: current controversies. Exp Neurol 223(2):282–293
Bate C, Williams A (2007) Squalestatin protects neurons and reduces the activation of cytoplasmic phospholipase A2 by Abeta(1–42). Neuropharmacology 53(2):222–231
Paris D, Townsend KP, Humphrey J, Obregon DF, Yokota K, Mullan M (2002) Statins inhibit A beta-neurotoxicity in vitro and A beta-induced vasoconstriction and inflammation in rat aortae. Atherosclerosis 161(2):293–299
Salins P, Shawesh S, He Y, Dibrov A, Kashour T, Arthur G et al (2007) Lovastatin protects human neurons against Abeta-induced toxicity and causes activation of beta-catenin–TCF/LEF signaling. Neurosci Lett 412(3):211–216
Chauhan NB, Siegel GJ, Feinstein DL (2004) Effects of lovastatin and pravastatin on amyloid processing and inflammatory response in TgCRND8 brain. Neurochem Res 29(10):1897–1911
Arvanitakis Z, Schneider JA, Wilson RS, Bienias JL, Kelly JF, Evans DA et al (2008) Statins, incident Alzheimer disease, change in cognitive function, and neuropathology. Neurology 70(19 Pt 2):1795–1802
Li Y, Tacey K, Doil L, van Luchene R, Garcia V, Rowland C et al (2004) Association of ABCA1 with late-onset Alzheimer's disease is not observed in a case-control study. Neurosci Lett 366(3):268–271
Rea TD, Breitner JC, Psaty BM, Fitzpatrick AL, Lopez OL, Newman AB et al (2005) Statin use and the risk of incident dementia: the Cardiovascular Health Study. Arch Neurol 62(7):1047–1051
Zandi PP, Sparks DL, Khachaturian AS, Tschanz J, Norton M, Steinberg M et al (2005) Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study. Arch Gen Psychiatry 62(2):217–224
Kandiah N, Feldman HH (2009) Therapeutic potential of statins in Alzheimer's disease. J Neurol Sci 283(1–2):230–234
Heverin M, Meaney S, Lutjohann D, Diczfalusy U, Wahren J, Bjorkhem I (2005) Crossing the barrier: net flux of 27-hydroxycholesterol into the human brain. J Lipid Res 46(5):1047–1052
Glebov K, Walter J (2012) Statins in unconventional secretion of insulin-degrading enzyme and degradation of the amyloid-beta peptide. Neurodegener Dis 10(1–4):309–312
Kuipers HF, Rappert AA, Mommaas AM, van Haastert ES, van der Valk P, Boddeke HW et al (2006) Simvastatin affects cell motility and actin cytoskeleton distribution of microglia. Glia 53(2):115–123
Pahan K, Sheikh FG, Namboodiri AM, Singh I (1997) Lovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophages. J Clin Invest 100(11):2671–2679
Shishehbor MH, Brennan ML, Aviles RJ, Fu X, Penn MS, Sprecher DL et al (2003) Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation 108(4):426–431
Tanaka K, Honda M, Takabatake T (2004) Anti-apoptotic effect of atorvastatin, a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, on cardiac myocytes through protein kinase C activation. Clin Exp Pharmacol Physiol 31(5–6):360–364
Zacco A, Togo J, Spence K, Ellis A, Lloyd D, Furlong S et al (2003) 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors protect cortical neurons from excitotoxicity. J Neurosci 23(35):11104–11111
Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM et al (2002) The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 420(6911):78–84
Eckert GP, Kirsch C, Mueller WE (2001) Differential effects of lovastatin treatment on brain cholesterol levels in normal and apoE-deficient mice. Neuroreport 12(5):883–887
Huttunen HJ, Havas D, Peach C, Barren C, Duller S, Xia W et al (2010) The acyl-coenzyme A: cholesterol acyltransferase inhibitor CI-1011 reverses diffuse brain amyloid pathology in aged amyloid precursor protein transgenic mice. J Neuropathol Exp Neurol 69(8):777–788
Bhattacharyya R, Kovacs DM (2010) ACAT inhibition and amyloid beta reduction. Biochim Biophys Acta 1801(8):960–965
Burns MP, Vardanian L, Pajoohesh-Ganji A, Wang L, Cooper M, Harris DC et al (2006) The effects of ABCA1 on cholesterol efflux and Abeta levels in vitro and in vivo. J Neurochem 98(3):792–800
Loane DJ, Washington PM, Vardanian L, Pocivavsek A, Hoe HS, Duff KE et al (2010) Modulation of ABCA1 by an LXR agonist reduces beta-amyloid levels and improves outcome after traumatic brain injury. J Neurotrauma 28(2):225–236
Riddell DR, Zhou H, Comery TA, Kouranova E, Lo CF, Warwick HK et al (2007) The LXR agonist TO901317 selectively lowers hippocampal Abeta42 and improves memory in the Tg2576 mouse model of Alzheimer's disease. Mol Cell Neurosci 34(4):621–628
Zelcer N, Khanlou N, Clare R, Jiang Q, Reed-Geaghan EG, Landreth GE et al (2007) Attenuation of neuroinflammation and Alzheimer's disease pathology by liver X receptors. Proc Natl Acad Sci USA 104(25):10601–10606
Shafaati M, O'Driscoll R, Bjorkhem I, Meaney S (2009) Transcriptional regulation of cholesterol 24-hydroxylase by histone deacetylase inhibitors. Biochem Biophys Res Commun 378(4):689–694
Qing H, He G, Ly PT, Fox CJ, Staufenbiel M, Cai F et al (2008) Valproic acid inhibits Abeta production, neuritic plaque formation, and behavioral deficits in Alzheimer's disease mouse models. J Exp Med 205(12):2781–2789
Grimm MO, Grimm HS, Patzold AJ, Zinser EG, Halonen R, Duering M et al (2005) Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nat Cell Biol 7(11):1118–1123
Grosgen S, Grimm MO, Friess P, Hartmann T (2010) Role of amyloid beta in lipid homeostasis. Biochim Biophys Acta 1801(8):966–974
Mohamed A, Saavedra L, Di Pardo A, Sipione S, Posse de Chaves E (2012) Beta-Amyloid inhibits protein prenylation and induces cholesterol sequestration by impairing SREBP-2 cleavage. J Neurosci 32(19):6490–6500
Acknowledgments
This was supported by grants from the Canadian Institutes of Health Research. MM is a recipient of the Alberta Innovates Health Research Studentship Award. SK and DW are recipients of Canada Research Chairs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maulik, M., Westaway, D., Jhamandas, J.H. et al. Role of Cholesterol in APP Metabolism and Its Significance in Alzheimer’s Disease Pathogenesis. Mol Neurobiol 47, 37–63 (2013). https://doi.org/10.1007/s12035-012-8337-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-012-8337-y