Abstract
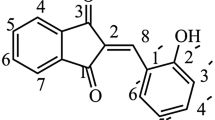
The studies on the determination of the characteristics of the amyloid fibril interaction with the dye were based on the analysis of the dependence of the ThT fluorescence intensity on its concentration in the solution containing the amyloid fibrils. In the present work, we revealed that this intuitive approach provided erroneous data. We propose a new approach which provides a means for characterizing the interaction of thioflavin T (ThT) with amyloid fibrils and for determining the binding stoichiometry and binding constants, absorption spectrum, molar extinction coefficient, and fluorescence quantum yield of the ThT bound to the sites of different binding modes of fibrils. The key point of this approach is sample preparation by equilibrium microdialysis. The efficiency of the proposed approach is demonstrated via the examination of the ThT binding to insulin and Aβ42 fibrils as well as to the native form of the Electrophorus electricus acetylcholinesterase. We show that the peculiarities of ThT interaction with amyloid fibrils depend on the amyloidogenic protein and on the binding mode. This approach is universal and can be used for the analysis of binding mechanism of any dye that interacts with its receptor. Therefore, the proposed approach represents an important addition to the existing arsenal of means for the diagnostics and therapy of the neurodegenerative diseases.






Similar content being viewed by others
References
Dobson CM (1999) Protein misfolding, evolution and disease. Trends Biochem Sci 24(9):329–332
Dobson CM (2003) Protein folding and misfolding. Nature 426(6968):884–890
LeVine H 3rd (1993) Thioflavine T interaction with synthetic Alzheimer’s disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci 2(3):404–410
LeVine H 3rd (1999) Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol 309:274–284
Naiki H, Higuchi K, Hosokawa M, Takeda T (1989) Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavin T1. Anal Biochem 177(2):244–249
Tycko R (2011) Solid-state NMR studies of amyloid fibril structure. Annu Rev Phys Chem 62:279–299
Tycko R (2004) Progress towards a molecular-level structural understanding of amyloid fibrils. Curr Opin Struct Biol 14(1):96–103
Sulatskaya AI, Kuznetsova IM, Maskevich AA, Uversky VN, Turoverov KK (2010) Non-radiative deactivation of the excited state of thioflavin T: dependence on solvent viscosity and temperature. PLoS One 5(10):e15385
Sulatskaya AI, Kuznetsova IM, Turoverov KK (2012) Interaction of thioflavin T with amyloid fibrils: fluorescence quantum yield of bound dye. J Phys Chem B 116(8):2538–2544
Groenning M (2010) Binding mode of Thioflavin T and other molecular probes in the context of amyloid fibrils-current status. J Chem Biol 3(1):1–18
Oravcova J, Bohs B, Lindner W (1996) Drug–protein binding sites. New trends in analytical and experimental methodology. J Chromatogr B Biomed Appl 677(1):1–28
Voropay ES, Samtsov MP, Kaplevsky KN, Maskevich AA, Stepuro VI, Povarova OI, Kuznetsova IM, Turoverov KK, Fink AL, Uversky VN (2003) Spectral properties of Thioflavin T and its complexes with amyloid fibrils. J Appl Spectrosc 70(6):868–874
Goers J, Permyakov SE, Permyakov EA, Uversky VN, Fink AL (2002) Conformational prerequisites for alpha-lactalbumin fibrillation. Biochemistry 41(41):12546–12551
Kayed R, Head E, Sarsoza F, Saing T, Cotman CW, Necula M, Margol L, Wu J, Breydo L, Thompson JL, Rasool S, Gurlo T, Butler P, Glabe CG (2007) Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener 2:18
De Ferrari GV, Mallender WD, Inestrosa NC, Rosenberry TL (2001) Thioflavin T is a fluorescent probe of the acetylcholinesterase peripheral site that reveals conformational interactions between the peripheral and acylation sites. J Biol Chem 276(26):23282–23287
Turoverov KK, Biktashev AG, Dorofeiuk AV, Kuznetsova IM (1998) A complex of apparatus and programs for the measurement of spectral, polarization and kinetic characteristics of fluorescence in solution. Tsitologiia 40(8–9):806–817
Sulatskaya AI, Kuznetsova IM, Turoverov KK (2011) Interaction of thioflavin T with amyloid fibrils: stoichiometry and affinity of dye binding, absorption spectra of bound dye. J Phys Chem B 115(39):11519–11524
Dzwolak W, Pecul M (2005) Chiral bias of amyloid fibrils revealed by the twisted conformation of Thioflavin T: an induced circular dichroism/DFT study. FEBS Lett 579(29):6601–6603
Uversky VN, Winter S, Lober G (1996) Use of fluorescence decay times of 8-ANS-protein complexes to study the conformational transitions in proteins which unfold through the molten globule state. Biophys Chem 60(3):79–88
Uversky VN, Winter S, Lober G (1998) Self-association of 8-anilino-1-naphthalene-sulfonate molecules: spectroscopic characterization and application to the investigation of protein folding. Biochim Biophys Acta 1388:133–142
Groenning M, Norrman M, Flink JM, van de Weert M, Bukrinsky JT, Schluckebier G, Frokjaer S (2007) Binding mode of Thioflavin T in insulin amyloid fibrils. J Struct Biol 159(3):483–497
Groenning M, Olsen L, van de Weert M, Flink JM, Frokjaer S, Jorgensen FS (2007) Study on the binding of Thioflavin T to beta-sheet-rich and non-beta-sheet cavities. J Struct Biol 158(3):358–369
Fodera V, Groenning M, Vetri V, Librizzi F, Spagnolo S, Cornett C, Olsen L, van de Weert M, Leone M (2008) Thioflavin T hydroxylation at basic pH and its effect on amyloid fibril detection. J Phys Chem B 112(47):15174–15181
Morimoto K, Kawabata K, Kunii S, Hamano K, Saito T, Tonomura B (2009) Characterization of type I collagen fibril formation using thioflavin T fluorescent dye. J Biochem 145(5):677–684
Sabate R, Lascu I, Saupe SJ (2008) On the binding of Thioflavin-T to HET-s amyloid fibrils assembled at pH 2. J Struct Biol 162(3):387–396
LeVine H 3rd (1997) Stopped-flow kinetics reveal multiple phases of thioflavin T binding to Alzheimer beta (1–40) amyloid fibrils. Arch Biochem Biophys 342(2):306–316
Lockhart A, Ye L, Judd DB, Merritt AT, Lowe PN, Morgenstern JL, Hong G, Gee AD, Brown J (2005) Evidence for the presence of three distinct binding sites for the thioflavin T class of Alzheimer’s disease PET imaging agents on beta-amyloid peptide fibrils. J Biol Chem 280(9):7677–7684
Ye L, Morgenstern JL, Gee AD, Hong G, Brown J, Lockhart A (2005) Delineation of positron emission tomography imaging agent binding sites on beta-amyloid peptide fibrils. J Biol Chem 280(25):23599–23604
Ye L, Velasco A, Fraser G, Beach TG, Sue L, Osredkar T, Libri V, Spillantini MG, Goedert M, Lockhart A (2008) In vitro high affinity alpha-synuclein binding sites for the amyloid imaging agent PIB are not matched by binding to Lewy bodies in postmortem human brain. J Neurochem 105(4):1428–1437
Sutharsan J, Dakanali M, Capule CC, Haidekker MA, Yang J, Theodorakis EA (2010) Rational design of amyloid binding agents based on the molecular rotor motif. Chem Med Chem 5(1):56–60
Krebs MR, Bromley EH, Donald AM (2005) The binding of thioflavin-T to amyloid fibrils: localisation and implications. J Struct Biol 149(1):30–37
Wu C, Biancalana M, Koide S, Shea JE (2009) Binding modes of thioflavin-T to the single-layer beta-sheet of the peptide self-assembly mimics. J Mol Biol 394(4):627–633
Biancalana M, Koide S (2010) Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim Biophys Acta 1804(7):1405–1412
Biancalana M, Makabe K, Koide A, Koide S (2009) Molecular mechanism of thioflavin-T binding to the surface of beta-rich peptide self-assemblies. J Mol Biol 385(4):1052–1063
Kitts CC, Vanden Bout DA (2009) Near-field scanning optical microscopy measurements of fluorescent molecular probes binding to insulin amyloid fibrils. J Phys Chem B 113(35):12090–12095
Turoverov KK, Kuznetsova IM, Maskevich AA, Stepuro VI, Kuzmitsky VA, Uversky VN (2007) ThT as an instrument for testing and investigation of amyloid and amyloid-like fibrils. Proc SPIE 6733:1–7
Maskevich AA, Stsiapura VI, Kuzmitsky VA, Kuznetsova IM, Povarova OI, Uversky VN, Turoverov KK (2007) Spectral properties of thioflavin T in solvents with different dielectric properties and in a fibril-incorporated form. J Proteome Res 6(4):1392–1401
Stsiapura VI, Maskevich AA, Kuzmitsky VA, Turoverov KK, Kuznetsova IM (2007) Computational study of thioflavin T torsional relaxation in the excited state. J Phys Chem A 111(22):4829–4835
Klunk WE, Wang Y, Huang GF, Debnath ML, Holt DP, Shao L, Hamilton RL, Ikonomovic MD, DeKosky ST, Mathis CA (2003) The binding of 2-(4′-methylaminophenyl)benzothiazole to postmortem brain homogenates is dominated by the amyloid component. J Neurosci 23(6):2086–2092
Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE (2003) Synthesis and evaluation of 11 C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem 46(13):2740–2754
Henriksen G, Hauser AI, Westwell AD, Yousefi BH, Schwaiger M, Drzezga A, Wester HJ (2007) Metabolically stabilized benzothiazoles for imaging of amyloid plaques. J Med Chem 50(6):1087–1089
Ono M (2009) Development of positron-emission tomography/single-photon emission computed tomography imaging probes for in vivo detection of beta-amyloid plaques in Alzheimer’s brains. Chem Pharm Bull (Tokyo) 57(10):1029–1039
Acknowledgments
This work was supported in part by the “Molecular and Cell Biology” Program of the Russian Academy of Sciences (KKT and VNU); Russian Foundation of Basic Research, grants #12-04-01651 (KKT) and #12-04-90022_Bel (KKT); and Dmitry Zimin’s Russian Charitable Foundation “Dynasty” (AIS). We are extremely grateful to Alexey V. Uversky for careful reading and editing this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kuznetsova, I.M., Sulatskaya, A.I., Uversky, V.N. et al. A New Trend in the Experimental Methodology for the Analysis of the Thioflavin T Binding to Amyloid Fibrils. Mol Neurobiol 45, 488–498 (2012). https://doi.org/10.1007/s12035-012-8272-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-012-8272-y