Abstract
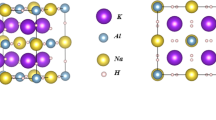
The potential of hydrogen as an energy carrier has been widely recognized due to concerns about climate change, environmental degradation and energy security. However, the storage and transportation of hydrogen remain significant challenges. Hydrides with a perovskite crystal structure can store large amounts of hydrogen in a small volume, and they are relatively easy to produce hydrogen. Among them, the ternary perovskite hydride NaMgH3 is distinguished by its relatively high theoretical hydrogen storage densities and reversibility of hydrogen absorption and desorption. In this study, first-principles calculations within the framework of density functional theory were employed to investigate the effect of substituting Na+ by K+ on the structural, electronic and hydrogen storage properties of Na1–xKxMgH3 (\(x \le 0.75 )\). The results show that the substitution of Na+ by K+ induces a slight decrease in the lattice parameters and an increase in the cell volume, and the MgH6 octahedron becomes more distorted, which is a good indicator of destabilization of the host material, ultimately leading to a decrease in decomposition temperature from 560.1 to 489.6 K, which is beneficial for hydrogen storage applications.




Similar content being viewed by others
References
Chai W S, Bao Y, Jin P, Tang G and Zhou L 2021 Renew. Sustain. Energy Rev. 147 111254
Liu W, Sun L, Li Z, Fujii M, Geng Y, Dong L et al 2020 Environ. Sci. Pollut. Res. 27 31092
Wang Z M, Li J J, Tao S, Deng J Q, Zhou H and Yao Q 2016 J. Alloys Compd. 660 402
Andrada-Chacón A, Alonso J A, Pomjakushin V and Sánchez-Benítez J 2017 J. Alloys Compd. 729 914
Tao S, Wang Z M, Li J J, Deng J Q, Zhou H and Yao Q R 2016 Mater. Sci. Forum 852 502
Sheppard D A, Paskevicius M and Buckley C E 2011 Chem. Mater. 23 4298
Satyapal S, Petrovic J, Read C, Thomas G and Ordaz G 2007 Catal. Today 120 246
Zhong H, Ouyang L Z, Ye J S, Liu J W, Wang H, Yao X D et al 2017 Energy Storage Mater. 7 222
Chaudhary A, Paskevicius M, Sheppard D A and Buckley C E 2015 J. Alloys Compd. 623 109
Kunkel N, Meijerink A, Springborg M and Kohlmann H 2014 J. Mater. Chem. C 2 4799
Li Y, Zhang L, Zhang Q, Fang F, Sun D, Li K et al 2014 J. Phys Chem C 118 23635
Wang Z, Tao S, Deng J, Zhou H and Yao Q 2017 Int. J. Hydrogen Energy 42 8554
Li Y, Mi Y, Chung J S and Kang S G 2018 Int. J. Hydrogen Energy 3 2232
Xiao X B, Tang B Y, Liao S Q, Peng L M and Ding W Jiang 2009 J. Alloys Compd. 474 522
Tao S, Wang Z and min, Wan Z zhen, Deng J Qiu, Zhou H and Yao Q, 2017 Int. J. Hydrogen Energy 42 3716
Blaha P, Schwarz K, Madsen G, Kvasnicka D and Luitz J 2001 WIEN2k (Technische Universität Wien, Austria)
Perdew J P, Burke K and Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
Tran F and Blaha P 2009 Phys. Rev. Lett. 102 5
Murnaghan F D 1924 J. Franklin Inst. 30 244
Vajeeston P, Ravindran P and Fjellv H 2007 J. Alloys Compd. 447 44
Contreras Vasquez L F, Liu Y, Paterakis C, Reed D and Book D 2017 Int. J. Hydrogen Energy 42 22589
Züttel A, Remhof A, Borgschulte A and Friedrichs O 2010 Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 368 3329
Boussami S, Khaldi C, Lamloumi J, Mathlouthi H and Takenouti H 2012 Electrochim. Acta 69 203
Li Y, Chung J S and Kang S G 2019 Comb. Sci 21 736
Chaib H, Mohammedi L, Benmebrouk L, Boukraa A, Daoudi B and Achouri A 2020 Int. J. Hydrogen Energy 45 28920
Zhou C, Bowman R C, Fang Z Z, Lu J, Xu L, Sun P et al 2019 ACS Appl. Mater. Interfaces 11 38868
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chami, R., Lekdadri, A., Baaddi, M. et al. First-principles insight of hydrogen storage properties of mixed perovskite hydrides Na1–xKxMgH3 (\(\user2{ x} \le 0.75\user2{ })\). Bull Mater Sci 46, 190 (2023). https://doi.org/10.1007/s12034-023-03035-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-023-03035-w