Abstract
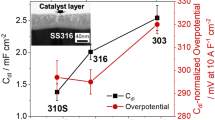
Stainless steel (SS) is attractive, easy to handle, cost-effective and offers several advantages (i.e., conductivity, durability, commercial availability) to make anodes. However, the presence of some iron oxides (mainly Fe2O3) on the SS surface improves electron transfer for potential use as an anode in sediment microbial fuel cells. Although several procedures are available to synthesize Fe2O3 on SS surfaces, most of them involve several careful steps, taking time (several hours or days) from start to finish. Fortunately, iron oxides can be synthesized on the SS surface quickly and very easily. Flame synthesis of iron oxides is a straightforward process, and it can be scalable. Using this procedure, two types of SS-grids 304 (wire diameters of 100 μm and 230 μm) material acquired from a common hardware store were flamed, forming Fe2O3 on their surface. Under different conditions (polished, polished then flamed, flamed) SS-grid (SSg) specimens were studied. All specimen surfaces were characterized by field emission scanning electron microscopy combined with X-rays chemical analysis. The chemical information of the iron oxides formed on the surface was obtained by X-ray diffractometer. The electrochemical responses of modified SSg pieces were assessed by cyclic voltammetry, and finally, their resistances were assessed by electrochemical impedance spectroscopy. An equivalent circuit was included to describe the electrode–electrolyte interface. The best electroactive area with small resistance in the electrode–electrolyte interface corresponds to the flamed SS grid (wire diameters of 100 mm).








Similar content being viewed by others
References
Prasad J and Tripathi R K 2020 J. Power Sources 450 227721
Hamdan H Z, Salam D A, Hari A R, Semerjian L and Saikaly P 2017 Sci. Total Environ. 575 1453
Li X, Zheng R, Zhang X, Liu Z, Zhu R, Zhang X et al 2019 J. Environ. Manag. 235 70
Sharma M, Aryal N, Sarma P M, Vanbroekhoven K, Lal B, Dominguez Benetton X et al 2013 Chem. Commun. 49 6495
Flimban S G A, Ismail I M I, Kim T and Oh S-E 2019 Energies 12 3390
Mukherjee P and Saravanan P 2019 ChemistrySelect 4 1601
Logan B E and Regan J M 2006 Trends Microbiol. 14 512
Hindatu Y, Annuar M S M and Gumel A M 2017 Renew. Sustain. Energy Rev. 73 236
Kerzenmacher S 2019 Adv. Biochem. Eng. Biotechnol. 167 135
Kumar G G, Sarathi V G S and Nahm K S 2013 Biosens. Bioelectron. 43 461
Scott K, Rimbu G A, Katuri K P, Prasad K K and Head I M 2007 Process Saf. Environ. Prot. 85 481
Le Huong T X, Bechelany M and Cretin M 2017 Carbon 122 564
Li S, Cheng C and Thomas A 2017 Adv. Mater. 29 1
Ouitrakul S, Sriyudthsak M, Charojrochkul S and Kakizono T 2007 Biosens. Bioelectron. 23 721
Tanisho S, Kamiya N and Wakao N 1989 J. Electroanal. Chem. 275 25
Mehanna M, Basséguy R, Délia M L and Bergel A 2009 Corros. Sci. 51 2596
Pocaznoi D, Calmet A, Etcheverry L, Erable B and Bergel A 2012 Energy Environ. Sci. 5 9645
Erable B and Bergel A 2009 Bioresour. Technol. 100 3302
Ketep S F, Bergel A, Calmet A and Erable B 2014 Energy Environ. Sci. 7 1633
Lamp J L, Guest J S, Naha S, Radavich K A, Love N G, Ellis M W et al 2011 J. Power Sources 196 5829
Tang Y, Bi X, Sun H, Fu J, Peng M and Zou H 2011 Adv. Mater. Res. 156–157 742
Dumas C, Basseguy R and Bergel A 2008 Electrochim. Acta 53 5235
Lowy D A, Tender L M, Zeikus J G, Park D H and Lovley D R 2006 Biosen. Bioelectron. 21 2058
Park I H, Christy M, Kim P and Nahm K S 2014 Biosens. Bioelectron 58 75
Okamoto A, Hashimoto K and Nakamura R 2012 Bioelectrochemistry 85 61
Ji J, Jia Y, Wu W, Bai L, Ge L and Gu Z 2011 Colloids Surfaces A Physicochem. Eng. Asp. 390 56
Peng X, Yu H, Wang X, Gao N, Geng L and Ai L 2013 J. Power Sources 223 94
Wang Q and Wang Y 2016 ACS Appl. Mater. Interfaces 8 10334
Long X, Cao X, Wang C, Liu S and Li X 2019 J. Electroanal. Chem. 855 113497
Betz G, Wehner G K, Toth L and Joshi A 1974 J. Appl. Phys. 45 5312
Saeki I, Konno H, Furuichi R, Nakurama T, Mabuchi K and Itoh M 1998 Corros. Sci. 40 191
Guo K, Donose B C, Soeriyadi A H, Prévoteau A, Patil S A, Freguia S et al 2014 Environ. Sci. Technol. 48 7151
Yamashita T, Ishida M, Asakawa S, Kanamori H, Sasaki H, Ogino A et al 2016 Biotechnol. Biofuels 9 1
Hauffe 1966 (eds) High temperature oxidation of metals (New York: John Wiley) https://doi.org/10.1002/maco.19670181017
Fujishima A, Zhang X and Tryk D A 2008 Surf. Sci. Rep. 63 515
Yuan X-Z, Song C, Wang H and Zhang J 2010 (eds) Electrochemical impedance spectroscopy in PEM fuel cells. Fundamentals and applications (London: Springer-Verlag). https://doi.org/10.1007/978-1-84882-846-9_2
Es-Souni M, Es-Souni M and Fischer-Brandies H 2002 Biomaterials 23 2887
Kumar S and Sankara Narayanan T S N 2011 J. Appl. Electrochem. 41 123
Parsons R 1990 Chem. Rev. 90 813
Selman J R and Lin Y P 1993 Electrochim. Acta 38 2063
Yin M-Y, Li Z, Xiao Z, Pang Y, Li Y-P and Shen Z-Y 2021 Trans. Nonferrous Met. Soc. China 31 1012
Solmaz R, Kardaş G, Çulha M, Yazıcı B and Erbil M 2008 Electrochim. Acta 53 5941
Guo X-P and Tomoe Y 1998 Corrosion 54 93
Guo K, Soeriyadi A H, Feng H, Prévoteau A, Patil A S, Gooding J J et al 2015 Bioresour. Technol. 195 46
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silva-Martínez, S., Pineda-Arellano, C.A., López-Sesenes, R. et al. Flame synthesis of Fe3O4/Fe2O3 on stainless steel grid surfaces to improve anodic electrochemical properties. Bull Mater Sci 46, 195 (2023). https://doi.org/10.1007/s12034-023-03034-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-023-03034-x