Abstract
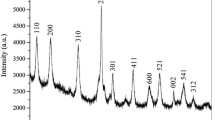
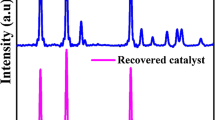
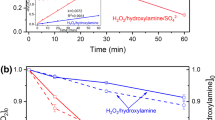
Manganese dioxide (α-MnO2) nanorods with diameters of about 5–15 nm and lengths of 100–150 nm were synthesized by a simple co-precipitation method, and the degradation mechanism of rhodamine B (RhB) at different pH levels was investigated. The α-MnO2 nanorods exhibited a high efficiency and rapid removal rate of RhB, which reached about 97.5% within 10 min when pH = 4 (and pH = 6.6) and 97.7% within 50 min when pH = 9 in the presence of H2O2. The results also indicated that a lower pH value is conducive to the blue shift of the characteristic peak of RhB dye and the attenuation of the characteristic peak intensity, which is mainly attributed to the combined effect of oxidative degradation and adsorption decolourization when pH is less than 9. Moreover, the α-MnO2 nanorod exhibits an excellent recyclability and catalytic stability. This research indicates that α-MnO2 nanorods have a potential application in practical dye pollutant treatment.









Similar content being viewed by others
References
Gagrani A, Zhou J W and Tsuzuki T 2018 Ceram. Int. 44 4694
Zhao G X, Li J X, Ren X M, Hu J, Hu W P and Wang X K 2013 RSC Adv. 3 12909
Sun H, Xu K L, Huang M J, Shang Y X, She P, Yin S Y et al 2015 Appl. Sur. Sci. 357 69
Anandkumar J and Mandal B 2011 J. Hazard. Mater. 186 1088
Yu K, Yang S G, He H, Sun C, Gu C G and Ju Y M 2009 J. Phys. Chem. A 113 10024
Thabit M, Liu H L, Zhang J and Wan B 2017 J. Environ. Sci. 60 53
Lee S Y, Kang D, Jeong S, Do H T and Kim J H 2020 ACS Omega 5 4233
Yu C L, Li G, Wei L F, Fan Q Z, Shu Q and Yu J C 2014 Catal. Today 224 154
Qu J Y, Shi L, He C X, Gao F, Li B B, Zhou Q et al 2014 Carbon 66 485
Sun M, Lan B, Lin T, Cheng G, Ye F, Yu L et al 2013 CrystEngComm 15 7010
Li K Z, Chen J J, Peng Y, Lin W C, Yan T and Li J H 2017 J. Mater. Chem. A 5 20911
He B B, Gao C, Zhao S F, Zeng X H, Li Y F, Yang R N et al 2019 J. Solid State Chem. 269 305
Boyom-Tatchemo F W, Devred F, Ndiffo-Yemeli G, Laminsi S and Gaigneaux E M 2020 Appl. Catal. B Environ. 260 118159
Husnain S M, Asim U, Yaqub A, Shahzad F and Abbas N 2020 New J. Chem. 44 6096
Khalid S and Cao C B 2017 New J. Chem. 41 5794
Maria-Hormigos R, Pacheco M, Jurado-Sánchez B and Escarpa A 2018 Environ. Sci. Nano 5 2993
Kang Y G, Yoon H, Lee C S, Kim E J and Chang Y S 2019 Water Res. 151 413
Huang J Z, Zhong S F, Dai Y F, Liu C C and Zhang H C 2018 Environ. Sci. Technol. 52 11309
Saputra E, Muhammad S, Sun H, Ang H M, Tade M and Wang S 2013 Sci. Technol. 47 5882
Meng Y T, Song W Q, Huang H, Ren Z, Chen S Y and Suib S L 2014 J. Am. Chem. Soc. 136 11452
Zhang B T, Cheng G, Ye W J, Zheng X Y, Liu H F, Sun M et al 2016 Dalton Trans. 45 18851
Gangwar D and Rath C 2021 Appl. Surf. Sci. 557 149693
Khan Y, Durrani S K, Mehmood M and Khan M R 2011 J. Mater. Res. 26 2268
Subramanian V, Zhu H W, Vajtai R, Ajayan P M and Wei B Q 2005 J. Phys. Chem. B 109 20207
Mishra K, Poudel T N, Basavegowda N and Lee Y R 2016 J. Catal. 344 273
Wang Y R, Zhang X F, He X, Zhang W, Zhang X X and Lu C H 2014 Carbohyd. Polym. 110 302
Mahamallik P, Saha S and Pal A 2015 Chem. Eng. J. 276 155
Panimalar S, Uthrakumar R, Selvi E T, Gomathy P, Inmozhi C, Kaviyarasu K et al 2020 Surf. Interfaces 20 100512
Sing K S W 1982 Pure Appl. Chem. 54 2201
John R E, Chandran A, Thomas M, Jose J and George K C 2016 Appl. Surf. Sci. 367 43
Huang C, Wang Y L, Gong M, Wang W, Mu Y and Hu Z H 2020 Purif. Technol. 230 115877
Watanabe T, Takizawa T and Honda K 1977 J. Phys. Chem. 81 1845
Han J, Wang M G, Cao S Y, Fang P, Lu S, Chen R et al 2013 J. Mater. Chem. A 1 13197
Pham A L T, Doyle F M and Sedlak D L 2012 Environ. Sci. Technol. 46 1055
Zhang W X, Yang Z H, Wang X, Zhang Y C, Wen X G and Yang S H 2006 Catal. Commun. 7 408
He Y, Jiang D B, Chen J, Jiang D Y and Zhang Y X 2018 J. Colloid Interf. Sci. 510 207
Khraisheh M A M, Al-Ghouti M A, Allen S J and Ahmad M N M 2004 Water Environ. Res. 76 2655
Deng J, Ge Y J, Tan C Q, Wang H Y, Li Q S, Zhou S Q J et al 2017 Chem. Eng. J. 330 1390
Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant number 51602140] and the Natural Science Foundation of Liaoning Province (grant number 2019-ZD-0189).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, S., Guan, A., Wang, J. et al. Highly efficient degradation of rhodamine B by α-MnO2 nanorods. Bull Mater Sci 45, 35 (2022). https://doi.org/10.1007/s12034-021-02620-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-021-02620-1