Abstract
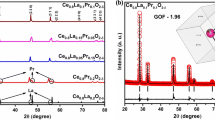
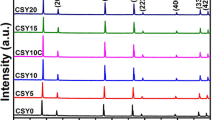
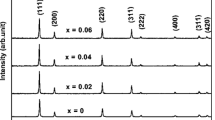
In this study, we aimed to investigate the structural, morphology and electrical properties of praseodymium and samarium co-doped ceria electrolytes, synthesized using the sol–gel method, for use as an electrolyte in low-temperature solid oxide fuel cell (LT-SOFC) applications. The X-ray diffraction result shows that all of the samples crystallized into a single-phase cubic fluorite form. The average relative densities of samples sintered at 1400°C is 97.9% of theoretical densities, indicating that they can be used as an electrolyte in LT-SOFC applications. A fascinating and maximum value of ionic conductivity \(1.94 \times 10^{ - 2}\) S cm–1 and least activation energy (Ea = 0.55 eV) were found for the composition Ce0.8Sm0.1Pr0.1O1.9 at a temperature of 500°C. The ionic conductivity and activation energies of compositions Ce0.85Sm0.1Pr0.05O1.925 and Ce0.9Sm0.05Pr0.05O1.95 found at 500°C were (\(1.41 \times 10^{ - 2}\) S cm–1, Ea = 0.59 eV) and (\(5.95 \times 10^{ - 3}\) S cm–1, Ea = 0.64 eV), respectively. Moreover, all the Praseodymium and samarium co-doped ceria (PrSDC) samples conduct at lower temperature of 300°C. All these results confirmed that praseodymium and samarium co-doped ceria can be useful as a solid electrolyte in LT-SOFC applications.






Similar content being viewed by others
References
Singhal S C and Kendall K 2003 High-temperature solid oxide fuel cells: Fundamentals, design and applications Elsevier Science 1 83
Mat M D, Liu X, Zhu Z and Zhu B 2007 Int. J. Hydrogen Energy 32 796
Park S, Vohs J M and Gorte R J 2000 Nature 404 265
Choudhury A, Chandra H and Arora A 2013 Ren. Sust. Energy Rev. 20 430
Wang F-Y, Chen S, Wang Q, Yu S and Cheng S 2004 Catal. Today 97 189
Mizutani Y, Tamura M, Kawai M and Yamamoto O 1994 Solid State Ion. 72 271
Taroco H A, Santos J A F, Domingues R Z and Matencio T 2011 Adv. Ceram Brasil 307 423
Brant M, Matencio T, Dessemond L and Domingues R 2006 Solid State Ion. 177 915
Jaiswal N, Tanwar K, Suman R, Kumar D, Upadhyay S and Parkash O 2019 J. Alloys Comp. 781 984
Gao Z, Mogni L V, Miller E C, Railsback J G and Barnett S A 2014 Energy Environ. Sci 9 1602
Wachsman E D and Lee K T 2011 Science 334 935
Zha S, Xia C and Meng G 2003 J. Power Sources 115 44
Arabacı A and Öksüzömer M F 2012 Ceram. Int. 38 6509
Altaf F, Batool R, Gill R, Abbas G, Raza R, Khan M A et al 2019 Ceram. Int. 45 10330
Suzuki T, Funahashi Y, Yamaguchi T, Fujishiro Y and Awano M 2009 Electrochem. 77 134
Maricle D, Swarr T and Karavolis S 1992 Solid State Ion. 52 173
Raharjo J, Setya Aninda R and Ami Lestari N 2017 J. Phys. Conf. Series 123 012077
Aydin F, Demir I and Mat M 2014 Int. J. Eng. Sci. Technol. 17 25
Liu M, Uba F and Liu Y 2020 J. Am. Ceram. Soc. 103 5325
Fan L, Zhu B, Su P-C and He C 2018 Nano Energy 45 148
Eressa L A and Rao P B 2020 Mater. Chem. Phys. 242 121914
Irfana S, Junsung A, Parvathi N, Srikar M, Sunaina P, Nivedithaa V et al 2018 Mater. Chem. Phys. 05 136
Bhabu K A, Theerthagiri J, Madhavan J, Balu T, Muralidharan G and Rajasekaran T R 2016 J. Mater. Sci.: Mater. Electron. 27 1566
Preethi S and Babu K S 2012 J. Alloys Comp. 792 1068
Singh N, Singh N K, Kumar D and Parkash O 2012 J. Alloys Comp. 519 129
Jaiswal N, Kumar D, Upadhyay S and Parkash O 2014 Ionics 20 45
Puente-Martínez D, Díaz-Guillén J, Montemayor S, Díaz-Guillén J, Burciaga-Díaz O, Bazaldúa-Medellín M et al 2020 Int. J. Hydrogen Energy 45 14062
Swatsitang E, Phokha S, Hunpratub S and Maensiri S 2016 Mater. Des. 108 27
Piumetti M, Bensaid S, Andana T, Dosa M, Novara C, Giorgis F et al 2017 Catalysts 7 174
Spanier J E, Robinson R D, Zhang F, Chan S-W and Herman I P 2001 Phys. Rev. B 64 245407
Eressa L A and Rao P B 2020 Chem. Tech. 12 28
Dos Santos M, Lima R, Riccardi C, Tranquilin R, Bueno P R, Varela J A et al 2008 Mater. Lett. 62 4509
Stojmenović M, Žunić M, Gulicovski J, Bajuk-Bogdanović D, Holclajtner-Antunović I, Dodevski V et al 2015 J. Mater. Sci. 50 3781
Jeyanthi C E, Siddheswaran R, Kumar P, Chinnu M K, Rajarajan K and Jayavel R 2015 Mater. Chem. Phys. 151 22
Shajahan I, Ahn J, Nair P, Medisetti S, Patil S, Niveditha V et al 2018 Mater. Chem. Phys. 216 136
Ahn K, Yoo D S, Prasad D H, Lee H-W, Chung Y-C and Lee J-H 2012 Chem. Mater. 24 4261
Dohcevic-Mitrovic Z, Radovic M, Scepanovic M, Grujic-Brojcin M, Popovic Z, Matovic B et al 2007 Appl. Phys. Lett. 91 203118
Jais A A, Ali S M, Anwar M, Somalu M R, Muchtar A, Isahak W N R W et al 2017 Ceram. Int. 43 8119
Ali S M, Anwar M, Abdalla A M, Somalu M R and Muchtar A 2017 Ceram. Int. 43 1265
Matović B, Stojmenović M, Pantić J, Varela A, Žunić M, Jiraborvornpongsa N et al 2014 Asian Ceram. Soc. 2 117
Xiaomin L, Qiuyue L, Lili Z and Xiaomei L 2015 J. Rare Earths 33 411
Singh V, Babu S, Karakoti A S, Agarwal A and Seal S 2010 J. Nanosci. Nanotech. 10 6495
Wang F-Y, Wan B-Z and Cheng S 2005 J. Solid State Electrochem. 9 168
Chockalingam R G K and Basu S 2014 J. Power Sources 250 80
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eressa, L.A., Rao, P.V.B. Electrical properties of praseodymium and samarium co-doped ceria electrolyte for low-temperature solid oxide fuel cell application. Bull Mater Sci 44, 255 (2021). https://doi.org/10.1007/s12034-021-02543-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-021-02543-x