Abstract
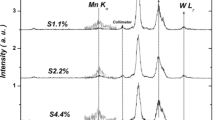
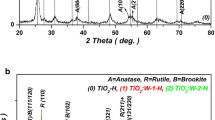
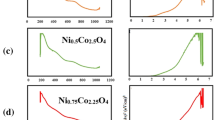
Tungsten oxide (W-oxide) nanoparticles doped and codoped with different transition-metal (TM) ions (Fe, Pt, Cu and Pd) were synthesized by hydrochloric acid-assisted precipitation. The synthesized powders were characterized by X-ray diffraction (XRD), diffuse reflectance spectroscopy (DRS) and magnetic characterization methods. The room temperature (RT) monoclinic (P21/n) structure founded for pristine \(\hbox {WO}_{3}\) nanopowder was converted into orthorhombic (Pbam) structure by Fe-doping, while codoping, (Fe–Pt) and (Fe–Cu) preserved the P21/n space group (SG) structure. It was found that the hydrogenation of the synthesized doped-samples corroded the crystallites without changing the crystalline SG structure. Moreover, controllable room temperature ferromagnetic (RT-FM) properties were created by hydrogenation of the codoped W-oxide samples. The oxygen vacancies-mediated ferromagnetic (FM) interaction could be responsible for the observed FM. The relative highest RT-FM energy was created with hydrogenated Fe–Pd codoped W-oxide. Therefore, Fe–Pd-codoped W-oxide nanopowder could be considered as a potential candidate for many applications involving partial FM properties, such as catalysts and optical phosphors.
Graphical Abstract






Similar content being viewed by others
References
Zhao P 2015 PhD thesis (Germany: University of Bremen)
El-Nouby M S 2014 PhD thesis (Osaka, Japan: Osaka University, OUKA)
Migas D B, Shaposhnikov V L and Borisenko V E 2010 J. Appl. Phys. 108 093714
Yan H, Zhang X, Zhou S, Xie X, Luo Y and Yu Y 2011 J. Alloys Compd. 509 L232
Lee K, Seo W S and Park J T 2003 J. Am. Chem. Soc. 125 3408
Lee S, Deshpande R, Parilla P A, Jones K M, To B, Mahan A H et al 2006 Adv. Mater. 18 763
Yamamoto S, Takano K, Inouye A and Yoshikawa M 2007 Nucl. Instrum. Meth. Phys. Res. Sect. B 262 29
Reyes L F, Hoel A, Saukko S, Hessler P, Lantto V and Granqvist C G 2006 Sens. Actuators B 117 128
Khatko V, Vallejos S, Calderer J, Gracia I, Cane C, Llobet E et al 2009 Sens. Actuators B 140 356
Castro-Hurtadoa I, Tavera T, Yurrita P, Perez N, Rodriguez A, Mandayo G G et al 2013 Appl. Surf. Sci. 276 229
Therese H A, Li J, Kolb U and Tremel W 2005 Solid State Sci. 7 67
Djaoued Y, Priya S and Balaji S 2008 J. Non-Cryst. Solids 354 673
Wang G, Ji Y, Huang X, Yang X, Gouma P and Dudley M 2006 J. Phys. Chem. B 110 23777
Yang B, Li H, Blackford M and Luca V 2006 Curr. Appl. Phys. 6 436
Hariharan V, Aroulmoji V, Prabakaran K, Gnanavel B, Parthibavarman M, Sathyapriya R et al 2016 J. Alloys Compd. 689 41
Kaminski A and Sarma S D 2002 Phys. Rev. Lett. 88 247202
Wolff P A, Bhatt R N and Durst A C 1996 J. Appl. Phys. 79 5196
Lewis E A, Le D, Murphy C J, Jewell A D, Mattewra M F G, Liriano M L et al 2012 J. Phys. Chem. C 116 25868
Pozzo M and Alfe D 2009 Int. J. Hydrog. Energy 34 1922
Wua E, Li W and Li J 2012 Int. J. Hydrog. Energy 37 1509
Zaluska A, Zaluski L and Strom-Olsen J O 1999 J. Alloys Compd. 288 217
Dakhel A A 2017 J. Supercond. Novel. Magn. Published online 24 November, https://doi.org/10.1007/s10948-017-4430-9
Luca L Introduction to diffraction and the Rietveld method (Corso: Laboratorio Scienza e Tecnologia dei Materiali) www.ing.unitn.it/~luttero/laboratoriomateriali/RietveldRefinements.pdf
Tanisaki S 1960 J. Phys. Soc. Jpn. 15 573
Woodward P M, Sleight A W and Vogt T 1995 J. Phys. Chem. Solids 56 1305
Barabanenkov Yu A, Zakharov N D, Zibrov I P, Filonenko V P, Werner P, Popov A I et al 1993 Acta Cryst. B 49 169
Shannon R D 1976 Acta Crystallogr. A 32 751
Kittel C 1996 Introduction to solid state physics (NY, USA: John Wiley & Sons)
Torrent J and Barron V 2002 Encyclopedia of surface and colloid science (NY, USA: Marcel Dekker Inc.)
Yaacob M H, Breedon M, Kalantar-Zadeh K and Wlodarski W 2009 Sens. Actuators B 137 115
Manfang M, Xinzhou M, Hua Z, Mao Y, Tao L, Shanming K et al 2017 J. Alloys Compd. 722 913
Matteo G, Carlo E B, Lucia C, Giovanni O, Cristiana D V and Gianfranco P 2015 J. Chem. Phys. 143 134702
Johansson M B, Baldissera G, Valyukh I, Persson C, Arwin H, Niklasson G et al 2013 J. Phys.: Condens. Matter 25 205502
Tauc J and Abeles F (eds) 1969 Optical properties of solids (Amsterdam: North Holland Publishing Co.)
Cole B, Marsen B, Miller E, Yan Y, To B, Jones K et al 2008 J. Phys. Chem. C 112 5213
Song H, Li Y, Lou Z, Xiao M, Hu L, Ye Z et al 2015 Appl. Catal. B 166–167 112
Aguir K, Lemire C and Lollman D B B 2002 Sens. Actuators B 84 1
Wang H, Dong X, Peng S, Dong L and Wang Y 2012 J. Alloys Compd. 527 204
Polaczek A, Pekala M and Obuszko Z 1994 J. Phys. Condens. Matter 6 7909
The University of the West Indies, Mona, Jamaica, Dept. of Chemistry, available: http://wwwchem.uwimona.edu.jm/spectra/MagMom.html (accessed on 8 September 2017)
Cheng W and Ma X 2009 J. Phys.: Conf. Ser. 152 012039
Yeganeh M, Shahtahmasebi N, Kompany A, Karimipour M, Razavi F, Nasralla N H S et al 2017 Physica B: Condens. Matter 511 89
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dakhel, A.A. Comparative study of structural, optical and magnetic properties of Fe–Pt, Fe–Cu and Fe–Pd-codoped \(\hbox {WO}_{3}\) nanocrystalline ceramics: effect of annealing in hydrogen atmosphere. Bull Mater Sci 41, 139 (2018). https://doi.org/10.1007/s12034-018-1667-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-018-1667-2