Abstract
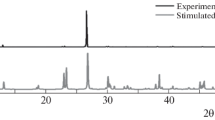
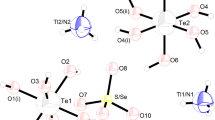
The new kröhnkite compound called potassium calcium-bis-hydrogen arsenate dihydrate K\(_{2}\)Ca(HAsO\(_{4})_{2}\cdot \)2H\(_{2}\)O was obtained by hydrothermal method and characterized by X-ray diffraction, infrared spectroscopy, Raman scattering, thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) analysis and optical (photoluminescence and absorption) properties. It crystallizes in the triclinic space group P\(\bar{1}\) and unit cell parameters \(a = 5.971(3)\) Å, \(b =6.634(3)\) Å, \(c = 7.856(4)\) Å, \(\alpha =104.532(9)\) \(^{\circ }\), \(\beta = 105.464(9)\) \(^{\circ }\) and \(\gamma = 109.698(9)\) \(^{\circ }\). The structure of K\(_{2}\)Ca(HAsO\(_{4})_{2}\cdot \)2H\(_{2}\)O built up from this infinite, (Ca(HAsO\(_{4})_{2}\)(H\(_{2}\)O)\(_{2})^{2+}\), was oriented along an axis resulting from the association of CaO\(_{6}\) octahedra alternating with each two HAsO\(_{4}\) tetrahedra by sharing corners. Each potassium atom links two adjacent chains by three oxygen atoms of HAsO\(_{4}\) tetrahedra. TGA and DSC have shown the absence of phase transition. The existence of vibrational modes corresponding to the kröhnkite is identified by the IR and Raman spectroscopies in the frequency ranges of 400–4000 and 20–4000 cm\(^{-1}\), respectively. The photoluminescence measurement show one peak at 507 nm, which is attributed to band–band (free electron–hole transitions) and (bound electron–hole transitions) emissions within the AsO\(_{4}\) inorganic part.






Similar content being viewed by others
References
Hawthrone F C S, Krivovichev V and Burns P C 2000 Rev. Miner. Geochem. 40 1
Fleck M, Kolitsch U and Hertweck B 2002 Z. Kristallogr. 217 435
Fleck M and Kolitsch U 2003 Z. Kristallogr. 218 553
Guillem G P, Cot L, Avinens C, Norbert A and Acad C R 1970 Sci. Ser. C 270 1870
Stoilova D, Wildner M, Marinova D and Georgiev M 2008 J. Mol. Struct. 889 12
Altomare A M, Burla C, Camalli M, Cascarano G L, Giacovazzo C, Guagliardi A et al 1999 SIR97 J. Appl. Crystallogr. 32 115
Sheldrick G M 1997 SHELXL-97, program for crystal structure refinement (Göttingen, Germany: University of Göttingen)
Farrugia L J 1999 J. Appl. Crystallogr. 32 837
Kolitsch U and Fleck M 2005 Z. Kristallogr. 220 31
Kolitsch U and Fleck M 2006 Eur. J. Miner. 18 471
Baur W H 1981 Interatomic distance predictions for computer simulation of crystal structures (eds) M O’Keeffe and A Navrotsky (New York: Academic Press) p 31
Brandenburg K 1998 Diamond, Version 2.0 (Bonn, Germany: Impact GbR) vol. II
Ferraris G 1970 Rend. Soc. Ital. Mineral. Petrol 26 589
Nakamoto K 1986 Infrared and Raman spectra of Inorganic and coordination compounds (New York: Wiley-Interscience)
Mihajlović T, Libowitzky E and Effenberger H 2004 J. Solid State Chem. 17 3963
Belhouchet M, Gargouri M, Mhiri T and Daoud A 2002 J. Phys. Chem. News 6 117
Debrus S, May M, Barycki J, Glowiak T, Barnes J A, Ratajaczak H et al 2004 J. Mol. Struct. 52 175
Nailiand H and Mhiri T 2001 J. Alloys Compd. 315 143
Kamoun S, Daoud A and Romain F 1991 J. Spectrochim. Acta 47 1051
Philip D and Druldhas B 1990 J. Raman Spectrosc. 21 211
Marchon B and Novak A 1985 J. Chem. Phys. 78 2105
Ohno N and Lockwood D J 1985 J. Chem. Phys. 83 4374
Choi B K and Kim J J 1985 J. Appl. Phys. 24 914
Baran J 1987 J. Mol. Struct. 162 211
Höppe A, Daub M and Oeckler O 2009 J. Solid State Sci. 11 1484
Wojciech Suchanek L, Shuk P, Byrappa K, Richard Riman E, Kevor S, TenHuisen F et al 2002 J. Biomater. 23 699
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ayadi, R., Lhoste, J., Dammak, T. et al. Crystal structure, thermal behaviour, vibrational spectroscopy and optical properties of new compounds K\(_{2}\)Ca(HAsO\(_{4}\))\(_{2}\cdot \)2H\(_{2}\)O with kröhnkite-type chain. Bull Mater Sci 41, 78 (2018). https://doi.org/10.1007/s12034-018-1581-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-018-1581-7