Abstract
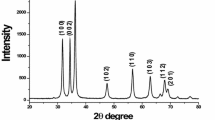
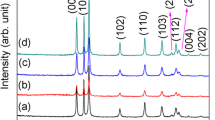
Uniform fine particles of zinc oxide were prepared in three different morphologies and sizes by the controlled precipitation process from aqueous solutions of zinc nitrate in the presence of ethylene glycol. Ammonium hydroxide solution was used as the precipitant. Composition of the reactant solution, pH and temperature significantly affected the particle uniformity with respect to shape and size. Uniformity in the particles morphological feature was achieved under a narrow set of experimental conditions. pH of the reactant solutions and isoelectric point of zinc oxide were considered the master variables, controlling the particle size. One of the batch of the as-prepared zinc oxide particles was calcined at \(750{^{\circ }}\hbox {C}\), which increased its crystallinity, changed its various lattice parameters, Zn–O bond length and preferred orientation of the crystal hkl planes. Calcination had little effect on the original morphology of the zinc oxide particles.







Similar content being viewed by others
References
Chandrappa K G, Venkatesha T V, Vathsala K and Shivakumara C 2010 J. Nanopart. Res. 12 2667
Suchanek L W 2009 J. Cryst. Growth 312 100
Zak A K, Razali R, Majid W A and Darroudi M 2011 Int. J. Nanomed. 6 1399
Lansdown A B, Mirastschijski U, Stubbs N, Scanlon E and Agren M S 2007 Wound Repair Regen. 15 2
Prasad A S 1995 Nutrition 11 39
Arslan K, Karahan O, Okus A, Unlu Y, Eryilmaz M A, Ay S et al. 2012 TJTES 18 376
Memarzadeh K, Sharili A S, Huang J, Rawlinson S C F and Allaker R P 2015 J. Biomed. Mater. Res. Part A 103A 81
He L H, Purton D G and Swain M V 2010 J. Dent. 38 290
Deng Y and Zhang H 2013 Int. J. Nanomed. 8 1835
Arakelova E R, Grigoryan S G, Arsenyan F G, Babayan N S, Grigoryan R M, Sarkisyan N K et al 2014 Int. J. Med. Health Biomed. Bioeng. Pharma. Eng. 8 33
Talebian N, Amininezhad S M and Doudi M 2013 J. Photochem. Photobiol. B 120 66
Farbod M and Jafarpoor E 2012 Mater. Lett. 85 47
Guo D, Wu C, Jiang H, Li Q, Wang X and Chen B 2008 J. Photochem. Photobiol. B 93 119
Goh E G, Xu X and McCormick P G 2014 Scr. Mater. 79 49
Tapiero H and Tew K D 2003 Biomed. Pharmacother. 57 399
Heidari A and Younesi H 2009 IJE Trans. B: Appl. 22 283
Zhang F, Wang X, Ai S, Sun Z, Wan Q, Zhu Z et al 2004 Anal. Chim. Acta 519 155
Umar A, Rahman M M, Al Hajry A and Hahn Y B 2009 Talanta 78 284
Li X, He G, Xiao G, Liu H and Wang M 2009 J. Colloid Interface Sci. 333 465
McBride R A, Kelly J M and McCormack D E 2003 J. Mater. Chem. 13 1
Ma M G, Zhu Y J, Cheng G F and Huang Y H 2008 Mater. Lett. 62 507
Nejati K, Rezvani Z and Pakizevand R 2011 Int. Nano Lett. 1 75
Shouli B, Liangyuan C, Dianqing L, Wensheng Y, Pengcheng Y, Zhiyong L et al 2010 Sens. Actuators B 146 129
Baruwati B, Kumar D K and Manorama S V 2006 Sens. Actuators B 119 676
Ada K, Sarıkaya Y, Alemdaroglu T and Onal M 2003 Ceram. Int. 29 513
Parekh B B, Vyas P M, Vasant S R and Joshi M J 2008 Bull. Mater. Sci. 31 143
Zhang S L, Byun H G, Lim J O, Huh J S and Lee W 2012 IEEE Sens. J. 12 3149
Boz I, Kaluza S, Boroglu M S and Muhler M 2012 Mater. Res. Bull. 47 1185
Marquez J A R, Rodriguez C M B, Herrera C M, Rosas E R, Angel O Z, Pozos O T et al 2011 Int. J. Electrochem. Sci. 6 4059
Wang Y, Tang W and Zhang L 2015 J. Mater. Sci. Technol. 31 175
Mazhdi M and Khani P H 2012 Int. J. Nano Dim. 2 233
Sofiani Z, Derkowska B, Dalasin S P, Wojdyla M, Dabos S S, Alaoui L M et al.2006 Opt. Commun. 267 433
Singhal S, Kaur J, Namgyal T and Sharma R 2012 Phys. B 407 1223
Acknowledgements
We are thankful to the National Centre of Excellence in Physical Chemistry, University of Peshawar, Peshawar 25120, Khyber Pakhtunkhwa, Pakistan, for facilitating this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akhtar, K., Zubair, N., Ikram, S. et al. Synthesis and characterization of ZnO nanostructures with varying morphology. Bull Mater Sci 40, 459–466 (2017). https://doi.org/10.1007/s12034-017-1386-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-017-1386-0