Abstract
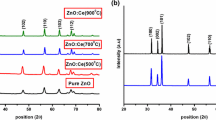
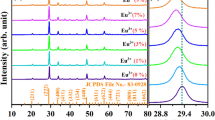
ZnO : Cr3+ (1 mol%) nanophosphor is synthesized by the wet chemical solution combustion method at the temperature of 400∘C. Powder X-ray diffraction results confirmed that Cr3+-doped and undoped ZnO nanophosphors exhibit hexagonal wurtzite structure. The average crystallite size calculated from Scherrer’s method is 25 nm for undoped and 14 nm for Cr3+-doped ZnO. The UV–visible absorption spectra shows red shift in Cr3+-doped ZnO. Photoluminescence studies of undoped ZnO show violet emission peak at 400 nm and blue emission peak at 447 nm. Cr3+-doped ZnO shows red emission peaks at 642, 694 and 746 nm, which are mainly attributed to spin forbidden transitions of 2Eg →\(^{4}\textit {A}_{2g}\) of Cr3+ ion in ZnO : Cr 3+ nanophosphor. Thermoluminescence (TL) studies recorded at a heating rate of 6∘C s −1 show two well-resolved glow peaks at 124 and 284∘C. It is found that the TL intensity increases with the gamma irradiation dose (500 Gy– 10 kGy).
Similar content being viewed by others
References
Willander M, Nur O, Zhao Q X, Yang L, Lorenz M, Cao B, Zuniga-Perez J, Czekalla C, Zimmermann G, Grundmann M, Bakin A, Behrends A, Al-Suleiman M, El-Shaer A, Mofo A C, Postels B, Waag A, Boukos N, Travlos A, Guinard J, Dang S and Le D 2009 Nanotechnology 20 332001
Pauporte T, Lincot D, Viana B and Pelle F 2006 J. Appl. Phys. Lett. 89 233112
Djurisic A B, Leung Y H, Tam K H, Hsu Y F, Ding L, Ge W K, Zhong Y C, Wong K S, Chan W K, Tam H L, Cheah K W, Kwok W M and Philips D L 2007 Nanotechnology 18 09570
Xu P S, Sun Y M, Shi C S, Xu F Q and Pan H B 2003 Nucl. Instrum. Methods: Phys. Res. Sect. B 19 286
Elilarassi R and Chandrasekaran G 2010 J. Optoelectron. Lett. 6 6
Chaun R, Kumar A and Choudhary R 2011 J. Optoelectron. Biomater. 3 17
Murugadoss G 2012 J. Mater. Sci. Technol. 28 487
Mazhdi M, Torkzadeh F and Mazhdi F M 2012 Int. J. Bioinorg. Hybrid Nanomater. 1 233
Yuhas B D, Zitoun D O, Pauzauskie P J, He R and Yang P 2006 Andew. Chem. Int. Ed. 45 420
Sharma P K, Datta R K and Pandey A C 2010 J. Colloid Interface Sci. 345 149
Gurkaynak T 2008 Altincekic and Boz I Bull. Mater. Sci. 31 619
Pandiyarajan T and Karthikeyan B 2012 J. Nanopart. Res. 14 647
Kumar P, Singh J, Parashar V, Singh K, Tiwari R S, Srivastava O N, Ramam K and Pandey A C 2012 Cryst. Eng. Commun. 14 1653
Schneider L, Zaitsev S V, Jin W, Kompch A, Winterer M and Acet Bacher M 2009 Nanotechnology 20 135604
Pan Z, Lu Y -Y and Liu F 2012 Nat. Mater. 11 58
Premkumar H B, Sunitha D V, Nagabhushana H, Sharma S C, Nagabhushana B M, Rao J L, Gupta K and Chakradhar R P S 2012 J. Spectrochim. Acta Part A 96 154
Hang, L, Zhioyong and Yan J 2011 Adv. Mater. Res. 306, 176
Ristić M, Musić S, Ivanda M and Popović S 2005 J. Alloys Compd. 397 L1
Tarwal N L, Jadhav P R, Vanalakar S A, Kalagi S S, Pawar R C, Shaikh J S, Mali S S, Dalavi D S, Shinde P S and Patil P S 2011 Powder Technol 8 185
Liu F, Yan W, Chuang Y -J, Zhen Z, Xie J and Pan Z 2013 Sci. Rep. 3 1554
Ekambaram S, Iikubo Y and Kudo A 2006 J. Alloys Compd. 433 237
Nagabhushana H, Nagabhushana B M, Kumar M, Premkumar H B, Shivakumara C and Chakradhar R P S 2010 Philos. Mag. 26 3567
Mote V D, Huse V R and Dole B N 2012 World J. Condens. Matter Phys. 2 208
Fan L, Song H, Li T, Yu L, Liu Z, Pan G, Lei Y, Bai X, Wang T, Zheng Z and Kong X 2007 J. Lumin. Res. 14 647
Shionoya S and Yen W H 1997 Phosphor handbook by Phosphor Research Society (Boca Raton, FL: CRC Press)
Jagannatha Reddy A, Kokila M K, Nagabhushana H, Chakradhar R P S, Shivakumara C, Rao J L and Nagabhushana B M 2011 J. Alloys Compd. 509 5349
Gupta A, Kumar S and Bhatti H S 2010 J. Mater. Sci.: Mater. Elect. 21 765
Jagannatha Reddy A 2012 Structural, luminescence and EPR studies of transition metal and rare earth ion doped zinc oxide nanomaterials PhD thesis (Bangalore University) Chapter 4, p 130
Lin B, Fu Z and Jia Y 2001 Appl. Phys. Lett. 79 943
Lee J D 2012 Concise inorganic chemistry(Wiley India) 5th edn, p 960
Cruz-Vazquez C, Orante-Barron V R, Grijalva-Monteverde H, Casta V M and Bernal R 2007 J. Mater. Lett. 61 1097
Secu C E and Sima M 2009 Opt. Mater. 3 876
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
PUSHPA, N., KOKILA, M.K., NAGABHUSHANA, B.M. et al. Red luminescence from ZnO : Cr3+ nanophosphors under visible excitation. Bull Mater Sci 38, 1359–1365 (2015). https://doi.org/10.1007/s12034-015-1021-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-015-1021-x