Abstract
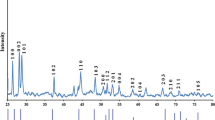
Cadmium sulfide (CdS) nanowires (NWs) were prepared by the solvothermal method using ethylenediamine as a solvent. Two sets of CdS NWs were synthesized at 160 and 200 °C for various reaction durations (3⋅5, 7, and 24 h). Scanning/tunneling electron microscopy was used to examine the surface morphology of the grown NWs. Their dimensions are found to depend on the reaction temperature and duration. The CdS NWs grown at 200 °C for all durations are longer than those prepared at 160 °C, with diameters ranging from 15 to 40 nm. A three-armed structure is exhibited by all the samples. The grown CdS NWs display a hexagonal wurtzite phase and grows along the \(\mathbf {\left \langle {001}\right \rangle }\) direction. The optical absorption of the grown NWs shows a sharp absorption edge with a blueshift, which indicates an expansion of the optical band gap. All prepared samples show two emission peaks in their photoluminescence spectra. The emission peak location depends on the reaction temperature and duration. The CdS NWs prepared at 160 °C show a sharp band–band emission compared with those prepared at 200 °C. Raman analysis indicates that the optical properties of the grown NWs are enhanced with increased temperature and reaction duration.








Similar content being viewed by others
References
Ahmad-Bitar R N 2000 Renew. Energ. 19 579
Arguello C A, Rousseau D L and Porto S P S 1969 Phys. Rev. 181 1351
Cao B L, Jiang Y, Wang C, Wang W H, Wang L Z, Niu M, Zhang W J, Li Y Q and Lee S T 2007 Adv. Funct. Mater. 17 1501
Chen S W and Wu J M 2011 Acta Mater. 59 841
Chen M, Xie Y, Lu J, Xiong Y, Zhang S, Qian Y and Liu X 2002 J. Mater. Chem. 12 748
Choi Y J, Park K S and Park J G 2010 Nanotechnol. 21 509901
Dalvand P and Mohammadi M R 2011 J. Nanopart. Res. 13 3011
Dalvand P, Mohammadi M R and Fray D J 2011 Matter. Lett. 65 1291
Datta A, Chavan P G, Sheini F J, More M A, Joag D S and Patra A 2009 Cryst. Growth Des. 9 4157
Jang J S, Joshi U A and Lee J S 2007 J. Phys. Chem. C 111 13280
Kar S and Chaudhuri S 2006 J. Phys. Chem. B 110 4542
Kar S, Santra S and Heinrich H 2008 J. Phys. Chem. C 112 4036
Li Q H, Gao T and Wang T H 2005 Appl. Phys. Lett. 86 193109
Li L, Wu P, Fang X, Zhai T, Dai L, Liao M, Koide Y, Wang H Q, Bando Y and Golberg D 2010 Adv. Mater. 22 3161
Ma R M, Wei X L, Dai L, Huo H D and Qin G G 2007 Nanotechnol. 18 205605
Mahdi M A, Hassan J J, Ng S S and Hassan Z 2012a Phys. E 44 1716
Mahdi M A, Hassan Z, Ng S S, Hassan J J and Mohd Bakhori S K 2012b Thin Solid Films 520 3477
Mahdi M A, Hassan J J, Ng S S and Hassan Z 2012c J. Cryst. Growth 359 43
Mahdi M A, Asmiet Ramzy, Hassan Z, Ng S S, Hassan J J and Kasim S K 2012d Chalcogen. Letts. 9 19
Mondal S P, Dhar A and Ray S K 2007a Mat Sci. Semicon. Proc. 10 185
Mondal S P, Das K, Dhar A and Ray S K 2007b Nanotechnol. 18 095606
Nien Y T, Chen P W and Chen I J 2008 J. Alloys Compd. 462 398
Nirmala Jothi N S, Chisty P D, Baby Suganthi A R, Ramalingam G and Sagayaraj P 2011 J. Crys. Growth 316 126
Owens F J and Poole C P 2008 The physics and chemistry of nanosolids (Hoboken, New Jersey, USA: John Wiley & Sons Inc.)
Pan A L, Liu R B, Yang Q, Zhu Y C, Yang G Z, Zou B S and Chen K Q 2005 J. Phys. Chem. B 109 24268
Phuruangrat A, Thongtoem T and Thongtoem S 2009 Mater. Lett. 63 1538
Phuruangrat A, Thongtem T and Thongten S 2010 Chalcogen. Letts. 7 605
Qingqing W, Gang X and Gaorong H 2005 J. Solid State Chem. 178 2680
Rai P, Song H M, Kim Y S, Song M K, Oh P R, Yoon J M and Yu Y T 2012 Mater. Lett. 68 90
Sadhu S, Chowdhury P S and Patra A 2008 J. Lumin. 128 1235
Thupakula U, Jena A, Khan A H, Dalui A and Acharya S 2012 J. Nanopart. Res. 14 701
Tsai C T, Chuu D S, Chen G L and Yang S L 1996 J. Appl. Phys. 79 9105
Wang Y W, Meng G W, Zhang L D, Liang C H and Zhang J 2002 Chem. Mater. 14 1773
Wang X, Liu W, Yang H, Li X, Li N, Shi R, Zhao H and Yu J 2011 Acta Mater. 59 1291
Xi Y, Hu C, Zheng C, Zhang H, Yang R and Tian Y 2010 Mater. Res. Bull. 45 1476
Xu D, Liu Z, Liang J and Qian Y 2005 J. Phys. Chem. B 109 14344
Yan S, Sun L, Qu P, Huang N, Song Y and Xiao Z 2009 J. Solid State Chem. 182 2941
Yingkai L, Xiangping Z, Dedong H and Hui W 2006 J. Mater. Sci. 41 6492
Zhou J, Zhao G, Yang J and Hano G 2011 J. Alloys Compd. 509 6731
Acknowledgements
The authors gratefully acknowledge the support of the Research University (RU) grant and the University Sains Malaysia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
MAHDI, M.A., HASSAN, J.J., KASIM, S.J. et al. Solvothermal growth of single-crystal CdS nanowires. Bull Mater Sci 37, 337–345 (2014). https://doi.org/10.1007/s12034-014-0655-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-014-0655-4