Abstract
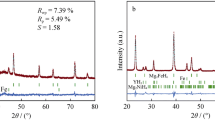
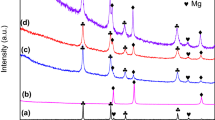
Two kinds of novel materials, Mg–1·6 mol%Ni–0·4 mol%NiO–2 mol%MCl (MCl = NbCl 5 , CrCl 3 ), along with Mg–1·6 mol%Ni–0·4 mol%NiO for comparison, were examined for their potential use in hydrogen storage applications, having been fabricated via cryomilling. The effects of NbCl 5 and CrCl 3 on hydrogen storage performance were investigated. A microstructure analysis showed that besides the main Mg and Ni phases, NiO and Mg 2 Ni phases were present in all samples. MgCl 2 was only found in halide-doped samples and NbO was only found in NbCl 5 -doped samples after ball milling. The particle size decreased significantly after 7 h of cryomilling. MgH 2 , Mg 2 NiH 4 and Mg 2 NiH0·3 were present in all the samples, while NbH 2 was only observed in the NbCl 5 -doped sample afterabsorption. The NbCl 5 -containing composite exhibited a low onset absorption temperature of 323 K, which was 10 K lower than that of the no-halide doped catalyst. It absorbed 5·32 wt% of hydrogen in 370 s at 623 K under 4 MPa hydrogen pressure and can absorb 90% of its full hydrogen capacity in 50 s. Having an onset desorption temperature of 483 K in vacuum, the NbCl 5 -containing composite desorbed hydrogen faster than the no-halide doped sample. The hydriding–dehydriding kinetics performance of the CrCl 3 -doped sample did not improve, but it did exhibit a lower onset desorption temperature of 543 K under 0·1 MPa, which was 20 K lower than that of the no-halide doped sample. NbO, NiO and NbH 2 played important roles in improving absorption and desorption performances.






Similar content being viewed by others
References
Aguey-Zinsou K-F and Ares-Fernández J-R 2008 Chem. Mater. 20 376
Barbir F 2009 Energy 34 308
Barkhordarian G, Klassen T and Bormann R 2003 Scr. Mater. 49 213
Berry G D, Pasternak A D, Rambach G D, Smith J R and Schock R N 1995 Energy 21 289
Czujko T, Varin R A, Chiu C and Wronski Z 2006 J. Alloys Compd 414 240
Fan M Q, Liu S S, Zhang Y, Zhang J, Sun L X and Xu F 2010 Energy 35 3417
Fuster V, Urretavizcaya G and Castro F J 2009 J. Alloys Compd 481 673
Hausdorf S, Balitalow F, Wolf G and Mertens F 2008 Int. J. Hydrogen Energy 33 608
Hong S H, Na Y S, Kwon S N, Bae J S and SongMY 2008 J. Alloys Compd 465 512
Ismail M, Zhao Y, Yu X B, Nevirkovets I P and Dou S X 2011 J. Alloys Compd 36 8327
Jain I P 2009 Int. J. Hydrogen Energy 34 7368
Jain I P, Chhagan Lal and Ankur Jain 2010 Int. J. Hydrogen Energy 35 5133
Jiang Kun, Xiao Xuezhang, Lixin Chen, Han Leyuan, Shouquan Li and Hongwei Ge 2012 J. Alloys Compd 539 103
Jin Seon-Ah, Shim Jae-Hyeok, Cho Young Whan and Yi Kyung- Woo 2007 J. Power Sources 172 859
Kalidindi Suresh Babu and Jagirdar Balaji R 2009 Inorg. Chem. 48 4524
Kalisvaart W P, Harrower C T, Haagsma J, Zahiri B, Luber E J and Ophus C 2010 Int. J. Hydrogen Energy 35 2091
Kwon S N, Baek S H, Mumm D R, Hong S H and Song M Y 2008 Int. J. Hydrogen Energy 33 4586
Li C, Peng P, Zhou D W and Wan L 2011 Int. J. Hydrogen Energy 36 14512
Ma L P, Kang X D, Dai H B, Liang Y, Fang Z Z, Wang P J and Cheng H M 2009 Acta Mater. 57 2250
Malka I E, Czujko T and Bystrzycki J 2010 Int. J. Hydrogen Energy 25 1706
Mao J, Guo Z, Yu X, Liu H, Wu Z and Ni J 2010 Int. J. Hydrogen Energy 35 4569
Neef H J 2009 Energy 34 327
Principi G, Agresti F, Massalena A and Russo S L 2009 Energy 34 2087
Sakintuna B, Lamari-Darkrim F and Hirscher M 2007 Int. J. Hydrogen Energy 32 1121
Tonus F, Fuster V, Urretavizcaya G, Castro F J and Bobet J L 2009 Int. J. Hydrogen Energy 34 3404
Varin R A and Jang M 2011 J. Alloys Compd 509 7134
Varin R A and Parviz R 2012 Int. J. Hydrogen Energy 37 9088
Xiong W, Li P, Xie D H, Zheng X P, Zheng C X and Qu X H 2009 Rare Metal Mat. Eng. 38 365
Zhai Fuqiang et al 2012 J. Phys. Chem. C 116 11939
Acknowledgements
This work was supported by the University of Science and Technology Beijing (USTB). (AV) acknowledges support from the National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
WAN, Q., LI, P., WANG, T. et al. NbCl5 and CrCl3 catalysts effect on synthesis and hydrogen storage performance of Mg–Ni–NiO composites. Bull Mater Sci 37, 77–82 (2014). https://doi.org/10.1007/s12034-014-0631-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-014-0631-z