Abstract
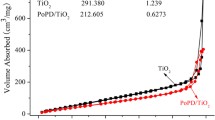
With an average size of 7 nm and good catalytic property under the natural light, TiO2/PS complex nanoparticles were successfully prepared through a novel two-step method (TSM) from TiCl4, used as both the catalyst for polymerization of styrene and Ti source, and styrene monomer and characterized by TG-DTA, XRD, IR, TEM and UV-Vis techniques. Its catalytic property was evaluated by the decolourization and degradation of dye MB solution under the natural light. From its TEM, the particles with an average size of 7 nm were observed without the separation of TiO2 and PS phases, i.e., TiO2/PS was hybrid material in nanosize scale. IR spectrum of TiO2/PS showed increase of unsaturated degree and growth of the group C=O on the chain of PS and Ti–O–C coordination bond between TiO2 and PS. The nanosize of the TiO2/PS complex particles and the conjugated structure and polar groups of PS were advantageous to good adsorptive property and strong interaction of PS and TiO2. And they brought multi-functions of inorganic and organic materials in the single material. Catalytic experiments indicated that the complex nanoparticles could catalytically degrade dye MB solution in 10 min under the natural light while P25 basically showed adsorptive property for MB molecules under the same conditions.








Similar content being viewed by others
References
Asahi R, Morikawa T, Ohwaki T, Aoki K and Taga Y 2001 Science 293 269
Chen H, Jin X L, Su B T, Fang Y J, Zhu K and Yang R Q 2000 Indian J. Chem. B A39 685
Cho S G and Choi W Y 2001 J. Photochem. Photobiol. A143 221
Duan C Y, Zhou J F, Wu Z S and Dang H X 2003 Chin. Acta Phys.-Chim. Sin. 19 1049
Gao Q Y, Zhang Y J and Yu X D 2001 Chin. Acta Polym. Sin. 3 329
Hoffmann M R, Martin S T, Choi W and Bahnemann D W 1995 Chem. Rev. 95 69
Jang S H, Han M G and Im S S 2000 Synth. Metal. 110 17
Ma Z Y, Min S X, She S X and Su B T 2005 Chin. J. Appl. Chem. 22 1137
Monredon S, Cellot A, Ribot F, Sanchez C, Armelao L, Gueneau L and Delattre L 2002 J. Mater. Chem. 12 2396
Ohno T, Mitsui T and Matsumura M 2003 Chem. Lett. 32 364
Salafsky J S, Lubberhuizen W H and Schropp R E I 1998 Chem. Phys. Lett. 290 297
Savenije T J, Warman J M and Goossens A 1998 Chem. Phys. Lett. 287 148
Savenije T J, Vermeulen M J W, De Haas M P and Warman J M 2000 Sol. Energy Mater. Sol. Cells 61 9
Sung-Suh H M, Choi J R, Hah H J, Koo S M and Bae Y C 2004 J.Photochem. Photobiol. A163 37
van Hal P A, Christiaans M P T, Wienk M M, Kroon J M and Janssen R A J 1999 J. Phys. Chem. B103 4352
Wang Y M, Liu S W, Lü M K, Wang S F, Gu F, Gai X Z, Cui X P and Pan J 2004 J. Mol. Catal. A215 137
Wang Y P, Yu J G, Zhao X J and Shi Z M 1998 China Environ. Sci. 18 244
Wang Z C, Li X J and Wang P 2003 China Environ. Sci. 23 535
Xie Y B and Yuan C W 2003 Appl. Catal. B46 251
Zhang H, Zong R L A and Zhu Y F 2009 J. Phys. Chem. C113 4605
Zhang J R and Gao L 2003 Chem. Lett. 32 458
Zhang L D and Mou J M 1994 Nanomaterials science (Shenyang: Liaoning Science and Technology Press) p. 171
Zhang Y H, Xiong G X, Yang W S and Fu X Z 2001 Chin. Acta Phys.-Chim. Sin. 17 273
Acknowledgements
This work was supported by the Natural Science Foundations of China (No.20963008) and Gansu Province (No. 0710RJZA119) and Shanxi Province (No. 0901-02 and No. 09JK802).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Su, B., Zhang, X., Ma, Z. et al. Catalytic property of TiO2/PS complex nanoparticles prepared via a novel TSM. Bull Mater Sci 33, 741–745 (2010). https://doi.org/10.1007/s12034-011-0140-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-011-0140-2