Abstract
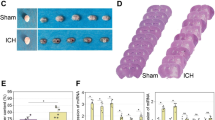
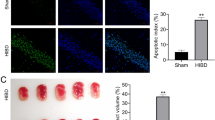
Intraventricular hemorrhage results in posthemorrhagic hydrocephalus (PHH). Neonatal hydrocephalus remains a challenging disease due to the high failure rate of all management strategies. We evaluated long noncoding RNA growth arrest-specific 5 (GAS5)-mediated network in neonatal hydrocephalus, providing a new direction for the treatment of hydrocephalus. The PHH model was constructed in neonatal rats after intracerebroventricular injection with GAS5, miR-325-3p, and chaperonin containing T-complex protein 1, subunit 8 (CCT8) plasmids, or oligonucleotides. Next, behavioral tests, measurement of serum inflammation, observation of brain tissue pathology, and calculation of hemoglobin and brain water contents were implemented. GAS5, miR-325-3p, and CCT8 expression, in combination with their interactions, was checked. As the results reported, collagenase infusion induced hydrocephalus, impairing neurological function, enhancing inflammation and neuronal apoptosis, and increasing hemoglobin and brain water contents. GAS5 and CCT8 were up-regulated, while miR-325-3p was down-regulated in hydrocephalic rats. Downregulating GAS5/CCT8 or upregulating miR-325-3p could inhibit inflammatory response and improve neurological function in young hydrocephalic rats. GAS5 promotes CCT8 expression through sponge adsorption of miR-325-3p. GAS5 silencing-mediated protections against hydrocephalus were counteracted by CCT8 overexpression. In summary, GAS5 aggravates neonatal hydrocephalus and inflammatory responses in a way of leasing miR-325-3p-involved regulation of CCT8.
Graphical Abstract






Similar content being viewed by others
Data availability
The data and materials used to support the findings of this study are available from the corresponding author.
References
Carter, C. S., Vogel, T. W., Zhang, Q., Seo, S., Swiderski, R. E., Moninger, T. O., Cassell, M. D., Thedens, D. R., Keppler-Noreuil, K. M., Nopoulos, P., Nishimura, D. Y., Searby, C. C., Bugge, K., & Sheffield, V. C. (2012). Abnormal development of NG2+PDGFR-α+ neural progenitor cells leads to neonatal hydrocephalus in a ciliopathy mouse model. Nature Medicine, 18(12), 1797–804.
James, H. E. (1992). Hydrocephalus in infancy and childhood. American Family Physician, 45(2), 733–742.
Henzi, R., Vío, K., Jara, C., Johanson, C. E., McAllister, J. P., Rodríguez, E. M., & Guerra, M. (2020). Neural stem cell therapy of foetal onset hydrocephalus using the HTx rat as experimental model. Cell and Tissue Research, 381(1), 141–161.
Kahle, K. T., Kulkarni, A. V., Limbrick, D. D., Jr., & Warf, B. C. (2016). Hydrocephalus in children. Lancet, 387(10020), 788–799.
Chen, Q., Tang, J., Tan, L., Guo, J., Tao, Y., Li, L., Chen, Y., Liu, X., Zhang, J. H., Chen, Z., & Feng, H. (2015). Intracerebral hematoma contributes to hydrocephalus after intraventricular hemorrhage via aggravating iron accumulation. Stroke, 46(10), 2902–8.
Wei, C. J., Li, Y. L., Zhu, Z. L., Jia, D. M., Fan, M. L., Li, T., Wang, X. J., Li, Z. G., & Ma, H. S. (2019). Inhibition of activator protein 1 attenuates neuroinflammation and brain injury after experimental intracerebral hemorrhage. CNS Neuroscience and Therapeutics, 25(10), 1182–1188.
Jiang, S., Cheng, S. J., Ren, L. C., Wang, Q., Kang, Y. J., Ding, Y., Hou, M., Yang, X. X., Lin, Y., Liang, N., & Gao, G. (2019). An expanded landscape of human long noncoding RNA. Nucleic Acids Research, 47(15), 7842–7856.
Seki, T., Yamagata, H., Uchida, S., Chen, C., Kobayashi, A., Kobayashi, M., Harada, K., Matsuo, K., Watanabe, Y., & Nakagawa, S. (2019). Altered expression of long noncoding RNAs in patients with major depressive disorder. Journal of Psychiatric Research, 117, 92–99.
Hou, J., Wang, L., Wu, Q., Zheng, G., Long, H., Wu, H., Zhou, C., Guo, T., Zhong, T., Wang, L., Chen, X., & Wang, T. (2018). Long noncoding RNA H19 upregulates vascular endothelial growth factor A to enhance mesenchymal stem cells survival and angiogenic capacity by inhibiting miR-199a-5p. Stem Cell Research and Therapy, 9, 1–109.
Chen, J. X., Wang, Y. P., Zhang, X., Li, G. X., Zheng, K., & Duan, C. Z. (2020). lncRNA Mtss1 promotes inflammatory responses and secondary brain injury after intracerebral hemorrhage by targeting miR-709 in mice. Brain Research Bulletin, 162, 20–29.
Schneider, C., King, R. M., & Philipson, L. (1988). Genes specifically expressed at growth arrest of mammalian cells. Cell, 54(6), 787–793.
Kino, T., Hurt, D. E., Ichijo, T., Nader, N., & Chrousos, G. P. (2010). Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Science Signaling, 3(107), ra8.
Wang, Y. N., Shan, K., Yao, M. D., Yao, J., Wang, J. J., Li, X., Liu, B., Zhang, Y. Y., Ji, Y., Jiang, Q., & Yan, B. (2016). Long noncoding RNA-GAS5: A novel regulator of hypertension-induced vascular remodeling. Hypertension, 68(3), 736–748.
Williams, G. T., Mourtada-Maarabouni, M., & Farzaneh, F. (2011). A critical role for non-coding RNA GAS5 in growth arrest and rapamycin inhibition in human T-lymphocytes. Biochemical Society Transactions, 39(2), 482–486.
Zhang, X. C., Gu, A. P., Zheng, C. Y., Li, Y. B., Liang, H. F., Wang, H. J., Tang, X. L., Bai, X. X., & Cai, J. (2019). YY1/LncRNA GAS5 complex aggravates cerebral ischemia/reperfusion injury through enhancing neuronal glycolysis. Neuropharmacology, 158, 107682.
Zhang, Z., Li, X., Chen, F., Li, Z., Wang, D., Ren, X., & Ma, H. (2021). Downregulation of LncRNA Gas5 inhibits apoptosis and inflammation after spinal cord ischemia-reperfusion in rats. Brain Research Bulletin, 168, 110–119.
Zhao, J. H., Wang, B., Wang, X. H., & Xu, C. W. (2019). Effect of lncRNA GAS5 on the apoptosis of neurons via the notch1 signaling pathway in rats with cerebral infarction. European Review for Medical and Pharmacological Sciences, 23(22), 10083–10091.
Feng, Z., Ye, L., Klebe, D., Ding, Y., Guo, Z. N., Flores, J. J., Yin, C., Tang, J., & Zhang, J. H. (2019). Anti-inflammation conferred by stimulation of CD200R1 via Dok1 pathway in rat microglia after germinal matrix hemorrhage. Journal of Cerebral Blood Flow and Metabolism, 39(1), 97–107.
Tang, J., Jila, S., Luo, T., Zhang, B., Miao, H., Feng, H., Chen, Z., & Zhu, G. (2022). C3/C3aR inhibition alleviates GMH-IVH-induced hydrocephalus by preventing microglia-astrocyte interactions in neonatal rats. Neuropharmacology, 205, 108927.
Yadav, A. K., Doran, S. F., Samal, A. A., Sharma, R., Vedagiri, K., Postlethwait, E. M., Squadrito, G. L., Fanucchi, M. V., Roberts, L. J., II., Patel, R. P., & Matalon, S. (2011). Mitigation of chlorine gas lung injury in rats by postexposure administration of sodium nitrite. American Journal of Physiology. Lung Cellular and Molecular Physiology, 300(3), L362–L369.
Tang, X. N., Berman, A. E., Swanson, R. A., & Yenari, M. A. (2010). Digitally quantifying cerebral hemorrhage using Photoshop and Image J. Journal of Neuroscience Methods, 190(2), 240–243.
Volpon Santos, M., da Silva Lopes, L., Machado, H. R., & Santos de Oliveira, R. (2019). Behavioral and biochemical features of the course and surgical treatment of experimental obstructive hydrocephalus in young rats. Developmental Neuroscience, 41(1–2), 34–43.
Mahaney, K. B., Buddhala, C., Paturu, M., Morales, D., Limbrick, D. D., Jr., & Strahle, J. M. (2020). Intraventricular hemorrhage clearance in human neonatal cerebrospinal fluid: Associations with hydrocephalus. Stroke, 51(6), 1712–1719.
Kulkarni, A. V., Schiff, S. J., Mbabazi-Kabachelor, E., Mugamba, J., Ssenyonga, P., Donnelly, R., Levenbach, J., Monga, V., Peterson, M., MacDonald, M., Cherukuri, V., & Warf, B. C. (2017). Endoscopic treatment versus shunting for infant hydrocephalus in Uganda. New England Journal of Medicine, 377(25), 2456–2464.
Shi, Y. H., He, X. W., Liu, F. D., Liu, Y. S., Hu, Y., Shu, L., Cui, G. H., Zhao, R., Zhao, L., Su, J. J., & Liu, J. R. (2019). Comprehensive analysis of differentially expressed profiles of long non-coding RNAs and messenger RNAs in kaolin-induced hydrocephalus. Gene, 697, 184–193.
Huang, F., Yi, J., Zhou, T., Gong, X., Jiang, H., & Yao, X. (2017). Toward understanding non-coding RNA roles in intracranial aneurysms and subarachnoid hemorrhage. Translational Neuroscience, 8, 54–64.
Hanjin, C., Tao, L., Pengfei, L., Ali, Y., Huajun, Z., Jiekun, L., Yang, W., & Tao, T. (2018). Altered long noncoding RNA and messenger RNA expression in experimental intracerebral hemorrhage—A preliminary study. Cellular Physiology and Biochemistry, 45(3), 1284–1301.
Chen-Roetling, J., Cao, Y., Peng, D., & Regan, R. F. (2019). Rapid loss of perihematomal cell viability in the collagenase intracerebral hemorrhage model. Brain Research, 1711, 91–96.
Ye, L., Gao, L., & Cheng, H. (2018). Inflammatory profiles of the interleukin family and network in cerebral hemorrhage. Cellular and Molecular Neurobiology, 38(7), 1321–1333.
Klebe, D., McBride, D., Flores, J. J., Zhang, J. H., & Tang, J. (2015). Modulating the immune response towards a neuroregenerative peri-injury milieu after cerebral hemorrhage. Journal of Neuroimmune Pharmacology, 10(4), 576–586.
Kim, J. M., Moon, J., Yu, J. S., Park, D. K., Lee, S. T., Jung, K. H., & Chu, K. (2019). Altered long noncoding RNA profile after intracerebral hemorrhage. Annals of Clinical Translational Neurology, 6(10), 2014–2025.
Xie, L., Wang, Y., & Chen, Z. (2021). LncRNA Blnc1 mediates the permeability and inflammatory response of cerebral hemorrhage by regulating the PPAR-γ/SIRT6/FoxO3 pathway. Life Sciences, 267, 118942.
Wen, J., Yang, C. Y., Lu, J., & Wang, X. Y. (2018). Ptprj-as1 mediates inflammatory injury after intracerebral hemorrhage by activating NF-κB pathway. European Review for Medical and Pharmacological Sciences, 22(9), 2817–2823.
Liu, T., Li, X., Cui, Y., Meng, P., Zeng, G., Wang, Y., & Wang, Q. (2021). Bioinformatics analysis identifies potential ferroptosis key genes in the pathogenesis of intracerebral hemorrhage. Frontiers in Neuroscience, 15, 661663.
Müller, A. H., Povlsen, G. K., Bang-Berthelsen, C. H., Kruse, L. S., Nielsen, J., Warfvinge, K., & Edvinsson, L. (2015). Regulation of microRNAs miR-30a and miR-143 in cerebral vasculature after experimental subarachnoid hemorrhage in rats. BMC Genomics, 16(1), 119.
Xi, T., Jin, F., Zhu, Y., Wang, J., Tang, L., Wang, Y., Liebeskind, D. S., & He, Z. (2017). MicroRNA-126-3p attenuates blood-brain barrier disruption, cerebral edema and neuronal injury following intracerebral hemorrhage by regulating PIK3R2 and Akt. Biochemical and Biophysical Research Communications, 494(1–2), 144–151.
Nie, H., Hu, Y., Guo, W., Wang, W., Yang, Q., Dong, Q., Tang, Y., Li, Q., & Tang, Z. (2020). miR-331-3p inhibits inflammatory response after intracerebral hemorrhage by directly targeting NLRP6. BioMed Research International, 2020, 6182464.
Yang, Y., Sun, B., Huang, J., Xu, L., Pan, J., Fang, C., Li, M., Li, G., Tao, Y., Yang, X., Wu, Y., Miao, P., Wang, Y., Li, H., Ren, J., Zhan, M., Fang, Y., Feng, X., & Ding, X. (2017). Up-regulation of miR-325-3p suppresses pineal aralkylamine N-acetyltransferase (Aanat) after neonatal hypoxia-ischemia brain injury in rats. Brain Research, 1668, 28–35.
Wu, X., Zhang, H., Chen, D., Song, Y., Qian, R., Chen, C., Mao, X., Chen, X., Zhang, W., Shao, B., Shen, J., Yan, Y., Wu, X., & Liu, Y. (2015). Up-regulation of CCT8 related to neuronal apoptosis after traumatic brain injury in adult rats. Neurochemical Research, 40(9), 1882–1891.
Acknowledgements
Not applicable.
Funding
National Natural Science Foundation of China (NO. (2019) 13, 81971217).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical Approval
All animal experiments were complied with the ARRIVE guidelines and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The experiments were approved by the Institutional Animal Care and Use Committee of Chongqing Medical University (Approval Number: 201706-AC59311).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original article has been corrected: Open access has been cancelled.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zou, B., Zhang, Q., Gan, H. et al. Long Noncoding RNA GAS5-Involved Progression of Neonatal Hydrocephalus and Inflammatory Responses. Mol Biotechnol (2024). https://doi.org/10.1007/s12033-024-01077-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12033-024-01077-x