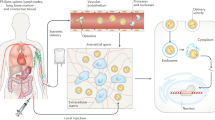
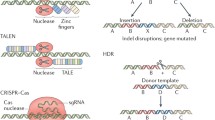
Abstract
The development of gene therapy based on genome editing has opened up new possibilities for the treatment of human genetic disorders. This field has developed rapidly over the past few decades, some genome editing-based therapies are already in phase 3 clinical trials. However, there are several challenges to be addressed before widespread adoption of gene editing therapy becomes possible. The main obstacles in the development of such therapy are safety and efficiency, so one of the biggest issues is the delivery of genetic constructs to patient cells. Approaches in genetic cargo delivery divide into ex vivo and in vivo, which are suitable for different cases. The ex vivo approach is mainly used to edit blood cells, improve cancer therapy, and treat infectious diseases. To edit cells in organs researches choose in vivo approach. For each approach, there is a fairly large set of methods, but, unfortunately, these methods are not universal in their effectiveness and safety. The focus of this article is to discuss the current status of in vivo and ex vivo delivery methods used in genome editing-based therapy. We will discuss the main methods employed in these approaches and their applications in current gene editing treatments under development.
Graphical Abstract




Similar content being viewed by others
Data availability
Not applicable.
References
Center for Biologics Evaluation and Research. (2020). What is gene therapy? FDA. Retrieved from https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/what-gene-therapy
Shahryari, A., Saghaeian Jazi, M., Mohammadi, S., Razavi Nikoo, H., Nazari, Z., Hosseini, E. S., et al. (2019). Development and clinical translation of approved gene therapy products for genetic disorders. Frontiers in Genetics, 10, 868. https://doi.org/10.3389/fgene.2019.00868
Gowing, G., Svendsen, S., & Svendsen, C. N. (2017). Ex vivo gene therapy for the treatment of neurological disorders. Progress in Brain Research, 230, 99–132. https://doi.org/10.1016/bs.pbr.2016.11.003
Mendell, J. R., Al-Zaidy, S. A., Rodino-Klapac, L. R., Goodspeed, K., Gray, S. J., Kay, C. N., et al. (2021). Current clinical applications of in vivo gene therapy with AAVs. Molecular Therapy: The Journal of the American Society of Gene Therapy, 29(2), 464–488. https://doi.org/10.1016/j.ymthe.2020.12.007
Liu, M., Rehman, S., Tang, X., Gu, K., Fan, Q., Chen, D., & Ma, W. (2019). Methodologies for improving HDR efficiency. Frontiers in Genetics, 9, 691. https://doi.org/10.3389/fgene.2018.00691
Kantor, A., McClements, M. E., & MacLaren, R. E. (2020). CRISPR-Cas9 DNA base-editing and prime-editing. International Journal of Molecular Sciences, 21(17), 6240. https://doi.org/10.3390/ijms21176240
Saha, S. K., Saikot, F. K., Rahman, Md. S., Jamal, M. A. H. M., Rahman, S. M. K., Islam, S. M. R., & Kim, K.-H. (2018). Programmable molecular scissors: Applications of a new tool for genome editing in biotech. Molecular Therapy. Nucleic Acids, 14, 212–238. https://doi.org/10.1016/j.omtn.2018.11.016
Silva, G., Poirot, L., Galetto, R., Smith, J., Montoya, G., Duchateau, P., & Pâques, F. (2011). Meganucleases and other tools for targeted genome engineering: Perspectives and challenges for gene therapy. Current Gene Therapy, 11(1), 11–27. https://doi.org/10.2174/156652311794520111
Kim, Y. G., Cha, J., & Chandrasegaran, S. (1996). Hybrid restriction enzymes: Zinc finger fusions to Fok I cleavage domain. Proceedings of the National Academy of Sciences of the United States of America, 93(3), 1156–1160. https://doi.org/10.1073/pnas.93.3.1156
Sakuma, T., & Yamamoto, T. (2023). Updated overview of TALEN construction systems. Methods in Molecular Biology (Clifton, N.J.), 2637, 27–39. https://doi.org/10.1007/978-1-0716-3016-7_2
Jiang, F., & Doudna, J. A. (2017). CRISPR-Cas9 Structures and Mechanisms. Annual Review of Biophysics, 46, 505–529. https://doi.org/10.1146/annurev-biophys-062215-010822
Liu, G., Lin, Q., Jin, S., & Gao, C. (2022). The CRISPR-Cas toolbox and gene editing technologies. Molecular Cell, 82(2), 333–347. https://doi.org/10.1016/j.molcel.2021.12.002
Morgan, M. A., Galla, M., Grez, M., Fehse, B., & Schambach, A. (2021). Retroviral gene therapy in Germany with a view on previous experience and future perspectives. Gene Therapy, 28(9), 494–512. https://doi.org/10.1038/s41434-021-00237-x
Themis, M., Forbes, S. J., Chan, L., Cooper, R. G., Etheridge, C. J., Miller, A. D., et al. (1998). Enhanced in vitro and in vivo gene delivery using cationic agent complexed retrovirus vectors. Gene Therapy, 5(9), 1180–1186. https://doi.org/10.1038/sj.gt.3300715
Nienhuis, A. W., Dunbar, C. E., & Sorrentino, B. P. (2006). Genotoxicity of retroviral integration in hematopoietic cells. Molecular Therapy: The Journal of the American Society of Gene Therapy, 13(6), 1031–1049. https://doi.org/10.1016/j.ymthe.2006.03.001
Carter, M., & Shieh, J. (2015). Chapter 11 – Gene delivery strategies. In M. Carter & J. Shieh (Eds.), Guide to research techniques in neuroscience (Second Edition) (pp. 239–252). Academic Press. https://doi.org/10.1016/B978-0-12-800511-8.00011-3
Dong, W., & Kantor, B. (2021). Lentiviral vectors for delivery of gene-editing systems based on CRISPR/Cas: Current state and perspectives. Viruses, 13(7), 1288. https://doi.org/10.3390/v13071288
Loewen, N., & Poeschla, E. M. (2005). Lentiviral vectors. Advances in Biochemical Engineering/Biotechnology, 99, 169–191. https://doi.org/10.1007/10_007
Wold, W. S. M., & Toth, K. (2013). Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Current Gene Therapy, 13(6), 421–433.
Syyam, A., Nawaz, A., Ijaz, A., Sajjad, U., Fazil, A., Irfan, S., et al. (2022). Adenovirus vector system: Construction, history and therapeutic applications. BioTechniques, 73(6), 297–305. https://doi.org/10.2144/btn-2022-0051
Pupo, A., Fernández, A., Low, S. H., François, A., Suárez-Amarán, L., & Samulski, R. J. (2022). AAV vectors: The Rubik’s cube of human gene therapy. Molecular Therapy: The Journal of the American Society of Gene Therapy, 30(12), 3515–3541. https://doi.org/10.1016/j.ymthe.2022.09.015
Lino, C. A., Harper, J. C., Carney, J. P., & Timlin, J. A. (2018). Delivering CRISPR: A review of the challenges and approaches. Drug Delivery, 25(1), 1234–1257. https://doi.org/10.1080/10717544.2018.1474964
Gehl, J. (2003). Electroporation: Theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiologica Scandinavica, 177(4), 437–447. https://doi.org/10.1046/j.1365-201X.2003.01093.x
Taylor, R. E., & Zahid, M. (2020). Cell penetrating peptides. Novel Vectors for Gene Therapy. Pharmaceutics, 12(3), 225. https://doi.org/10.3390/pharmaceutics12030225
Liu, J., Gaj, T., Patterson, J. T., Sirk, S. J., & Barbas, C. F. (2014). Cell-penetrating peptide-mediated delivery of TALEN proteins via bioconjugation for genome engineering. PLoS ONE, 9(1), e85755. https://doi.org/10.1371/journal.pone.0085755
Wahane, A., Waghmode, A., Kapphahn, A., Dhuri, K., Gupta, A., & Bahal, R. (2020). Role of lipid-based and polymer-based non-viral vectors in nucleic acid delivery for next-generation gene therapy. Molecules, 25(12), 2866. https://doi.org/10.3390/molecules25122866
Kazemian, P., Yu, S.-Y., Thomson, S. B., Birkenshaw, A., Leavitt, B. R., & Ross, C. J. D. (2022). Lipid-nanoparticle-based delivery of CRISPR/Cas9 genome-editing components. Molecular Pharmaceutics, 19(6), 1669–1686. https://doi.org/10.1021/acs.molpharmaceut.1c00916
Duan, L., Ouyang, K., Xu, X., Xu, L., Wen, C., Zhou, X., et al. (2021). Nanoparticle delivery of CRISPR/Cas9 for genome editing. Frontiers in Genetics, 12, 673286. https://doi.org/10.3389/fgene.2021.673286
Sun, W., Lu, Y., & Gu, Z. (2015). Rolling circle replication for engineering drug delivery carriers. Therapeutic Delivery, 6(7), 765–768. https://doi.org/10.4155/tde.15.27
Sun, W., Ji, W., Hall, J. M., Hu, Q., Wang, C., Beisel, C. L., & Gu, Z. (2015). Efficient delivery of CRISPR-Cas9 for genome editing via self-assembled DNA nanoclews. Angewandte Chemie (International ed. in English), 54(41), 12029–12033. https://doi.org/10.1002/anie.201506030
Ex Vivo & In Vivo Gene Therapy Techniques. (n.d.). Retrieved August 25, 2023, from https://www.thegenehome.com/how-does-gene-therapy-work/techniques
Worgall, S., & Crystal, R. G. (2020). Chapter 28 – Gene therapy. In R. Lanza, R. Langer, J. P. Vacanti, & A. Atala (Eds.), Principles of tissue engineering (Fifth Edition) (pp. 493–518). Academic Press. https://doi.org/10.1016/B978-0-12-818422-6.00029-0
Maier, D. A., Brennan, A. L., Jiang, S., Binder-Scholl, G. K., Lee, G., Plesa, G., et al. (2013). Efficient clinical scale gene modification via zinc finger nuclease-targeted disruption of the HIV co-receptor CCR5. Human Gene Therapy, 24(3), 245–258. https://doi.org/10.1089/hum.2012.172
Tebas, P., Jadlowsky, J. K., Shaw, P. A., Tian, L., Esparza, E., Brennan, A. L. et al. (n.d.). CCR5-edited CD4+ T cells augment HIV-specific immunity to enable post-rebound control of HIV replication. The Journal of Clinical Investigation, 131(7), e144486. https://doi.org/10.1172/JCI144486
Labanieh, L., Majzner, R. G., & Mackall, C. L. (2018). Programming CAR-T cells to kill cancer. Nature Biomedical Engineering, 2(6), 377–391. https://doi.org/10.1038/s41551-018-0235-9
Ramachandran, V., Kolli, S. S., & Strowd, L. C. (2019). Review of graft-versus-host disease. Dermatologic Clinics, 37(4), 569–582. https://doi.org/10.1016/j.det.2019.05.014
allo-20191231. (n.d.). Retrieved August 25, 2023, from https://www.sec.gov/Archives/edgar/data/1737287/000173728720000012/allo-20191231.htm
FDA Lifts Clinical Hold on MELANI-01 Study evaluating cellectis’ product candidate UCARTCS1 in multiple myeloma | cellectis. (n.d.). Retrieved August 25, 2023, from https://www.cellectis.com/en/press/fda-lifts-clinical-hold-on-melani-01-study-evaluating-cellectis-product-candidate-ucartcs1-in-multiple-myeloma
Sugita, M., Galetto, R., Zong, H., Ewing-Crystal, N., Trujillo-Alonso, V., Mencia-Trinchant, N., et al. (2022). Allogeneic TCRαβ deficient CAR T-cells targeting CD123 in acute myeloid leukemia. Nature Communications, 13, 2227. https://doi.org/10.1038/s41467-022-29668-9
MacLeod, D. T., Antony, J., Martin, A. J., Moser, R. J., Hekele, A., Wetzel, K. J., et al. (2017). Integration of a CD19 CAR into the TCR alpha chain locus streamlines production of allogeneic gene-edited CAR T cells. Molecular Therapy: The Journal of the American Society of Gene Therapy, 25(4), 949–961. https://doi.org/10.1016/j.ymthe.2017.02.005
McCreedy, B. J., Senyukov, V. V., & Nguyen, K. T. (2018). Off the shelf T cell therapies for hematologic malignancies. Best Practice & Research. Clinical Haematology, 31(2), 166–175. https://doi.org/10.1016/j.beha.2018.03.001
Jacobson, C. A., Herrera, A. F., Budde, L. E., DeAngelo, D. J., Heery, C., Stein, A., et al. (2019). Initial findings of the phase 1 trial of PBCAR0191, a CD19 targeted allogeneic CAR-T cell therapy. Blood, 134(Supplement_1), 4107. https://doi.org/10.1182/blood-2019-128203
Huang, C., Li, Q., & Li, J. (2022). Site-specific genome editing in treatment of inherited diseases: Possibility, progress, and perspectives. Medical Review, 2(5), 471–500. https://doi.org/10.1515/mr-2022-0029
Frangoul, H., Altshuler, D., Cappellini, M. D., Chen, Y.-S., Domm, J., Eustace, B. K., et al. (2021). CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. The New England Journal of Medicine, 384(3), 252–260. https://doi.org/10.1056/NEJMoa2031054
Abstract Details | ASGCT Annual Meeting. (n.d.). Retrieved August 25, 2023, from https://annualmeeting.asgct.org/abstracts/abstract-details?abstractId=14541
Fang, R., Yuan, P., Yu, L., Yang, H., Liu, J., Shi, J., et al. (2019). Manufacturing scale-up and preclinical development of ET-01, autologous CD34+ cells with the BCL11A erythroid enhancer. Edited By CRISPR/Cas9, for Patients with β-Thalassemia Major. Blood, 134(Supplement_1), 965. https://doi.org/10.1182/blood-2019-126499
UC consortium launches first clinical trial u | EurekAlert! (n.d.). Retrieved August 25, 2023, from https://www.eurekalert.org/news-releases/689894
Xu, L., Wang, J., Liu, Y., Xie, L., Su, B., Mou, D., et al. (2019). CRISPR-edited stem cells in a patient with HIV and acute lymphocytic leukemia. The New England Journal of Medicine, 381(13), 1240–1247. https://doi.org/10.1056/NEJMoa1817426
Cossette, D., Aiyer, S., Kimball, C., Luby, C., Zarate, J., Eng, J., et al. (2021). Clinical-scale production and characterization of Ntla-5001 – A novel approach to manufacturing CRISPR/Cas9 engineered T cell therapies. Blood, 138, 3881. https://doi.org/10.1182/blood-2021-153775
Wang, Z., Li, N., Feng, K., Chen, M., Zhang, Y., Liu, Y., et al. (2021). Phase I study of CAR-T cells with PD-1 and TCR disruption in mesothelin-positive solid tumors. Cellular and Molecular Immunology, 18(9), 2188–2198. https://doi.org/10.1038/s41423-021-00749-x
Harmatz, P., Prada, C. E., Burton, B. K., Lau, H., Kessler, C. M., Cao, L., et al. (2022). First-in-human in vivo genome editing via AAV-zinc-finger nucleases for mucopolysaccharidosis I/II and hemophilia B. Molecular Therapy, 30(12), 3587–3600. https://doi.org/10.1016/j.ymthe.2022.10.010
Editas Medicine Announces Clinical Data Demonstrating Proof of Concept of EDIT-101 from Phase 1/2 BRILLIANCE Trial | Editas Medicine. (n.d.). Retrieved August 25, 2023, from https://ir.editasmedicine.com/news-releases/news-release-details/editas-medicine-announces-clinical-data-demonstrating-proof
Gillmore, J. D., Gane, E., Taubel, J., Kao, J., Fontana, M., Maitland, M. L., et al. (2021). CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. The New England Journal of Medicine, 385(6), 493–502. https://doi.org/10.1056/NEJMoa2107454
Intellia Therapeutics Presents New Interim Data from First-in-Human Study of NTLA-2002 for the Treatment of Hereditary Angioedema (HAE) at the American College of Allergy, Asthma & Immunology 2022 Annual Scientific Meeting - Intellia Therapeutics. (n.d.). Retrieved August 25, 2023, from https://ir.intelliatx.com/news-releases/news-release-details/intellia-therapeutics-presents-new-interim-data-first-human
Chen, Z., Ling, L., Shi, X., Li, W., Zhai, H., Kang, Z., et al. (2021). Microinjection of antisense oligonucleotides into living mouse testis enables lncRNA function study. Cell & Bioscience, 11(1), 213. https://doi.org/10.1186/s13578-021-00717-y
Shen, X., Beasley, S., Putman, J. N., Li, Y., Prakash, T. P., Rigo, F., et al. (2019). Efficient electroporation of neuronal cells using synthetic oligonucleotides: identifying duplex RNA and antisense oligonucleotide activators of human frataxin expression. RNA, 25(9), 1118–1129. https://doi.org/10.1261/rna.071290.119
Roberts, T. C., Langer, R., & Wood, M. J. A. (2020). Advances in oligonucleotide drug delivery. Nature Reviews. Drug Discovery, 19(10), 673–694. https://doi.org/10.1038/s41573-020-0075-7
Yang, S., Wang, D., Sun, Y., & Zheng, B. (2019). Delivery of antisense oligonucleotide using polyethylenimine-based lipid nanoparticle modified with cell penetrating peptide. Drug Delivery, 26(1), 965–974. https://doi.org/10.1080/10717544.2019.1667453
Ding, Y., Jiang, Z., Saha, K., Kim, C. S., Kim, S. T., Landis, R. F., & Rotello, V. M. (2014). Gold nanoparticles for nucleic acid delivery. Molecular Therapy: The Journal of the American Society of Gene Therapy, 22(6), 1075–1083. https://doi.org/10.1038/mt.2014.30
Acknowledgements
All the figures were created with BioRender.com
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Volodina, O., Smirnikhina, S. The Future of Gene Therapy: A Review of In Vivo and Ex Vivo Delivery Methods for Genome Editing-Based Therapies. Mol Biotechnol (2024). https://doi.org/10.1007/s12033-024-01070-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12033-024-01070-4