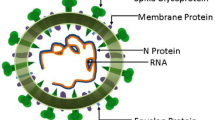
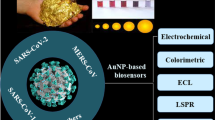
Abstract
Humans contract the Chikungunya virus (CHIKV), an alphavirus transmitted by mosquitoes that induces acute and chronic musculoskeletal discomfort and fever. Millions of cases of the disease have been attributed to CHIKV in the Indian Ocean region since 2004, and the virus has since spread to Europe, the Middle East, and the Pacific. The exponential proliferation of CHIKV in recent times underscores the critical nature of implementing preventative measures and exploring potential control strategies. The principal laboratory test employed to diagnose infection in serum samples collected over six days after the onset of symptoms is the detection of CHIKV or viral RNA. Although two commercially available real-time reverse transcription–polymerase chain reaction products exist, data on their validity are limited. A diagnostic instrument that is rapid, sensitive, specific, and cost-effective is, therefore an absolute necessity, particularly in developing nations. Biosensors have demonstrated considerable potential in the realm of pathogen detection. The rapid and sensitive detection of viruses has been facilitated by the development of numerous types of biosensors, including affinity-based nano-biosensors, graphene affinity-based biosensors, optical nano-biosensors, surface Plasmon Resonance-based optical nano-biosensors, and electrochemical nano-biosensors. Furthermore, the utilization of nanomaterials for signal extension, including but not limited to gold and silver nanoparticles, quantum dots, and iron oxide NPs, has enhanced the precision and sensitivity of biosensors. The developed innovative diagnostic method is time-efficient, precise, and economical; it can be implemented as a point-of-care device. The technique may be implemented in diagnostic laboratories and hospitals to identify patients infected with CHIKV. Throughout this article, we have examined a multitude of CHIKV nano-biosensors and their respective properties. Following a discussion of representative nanotechnologies for biosensors, numerous NPs-assisted CHIKV nano-biosensors are summarized in this article. As a result, we anticipate that this review will furnish a significant foundation for advancing innovative CHIKV nano-biosensors.
Graphical Abstract




Similar content being viewed by others
Data Availability
Not applicable.
References
Schmidt, C., Haefner, E., Gerbeth, J., Beissert, T., Sahin, U., Perkovic, M., & Schnierle, B. S. (2022). A taRNA vaccine candidate induces a specific immune response that protects mice against Chikungunya virus infections. Molecular Therapy-Nucleic Acids, 28, 743–754.
Weaver, S. C., & Forrester, N. L. (2015). Chikungunya: Evolutionary history and recent epidemic spread. Antiviral Research, 120, 32–39.
Montalvo Zurbia-Flores, G., Reyes-Sandoval, A., & Kim, Y. C. (2023). Chikungunya virus: Priority pathogen or passing trend? Vaccines, 11(3), 568.
Hakim, M. S., Annisa, L., Gazali, F. M., & Aman, A. T. (2022). The origin and continuing adaptive evolution of chikungunya virus. Archives of Virology, 167(12), 2443–2455.
Abdelnabi, R., Neyts, J., & Delang, L. (2017). Chikungunya virus infections: Time to act, time to treat. Current Opinion in Virology, 24, 25–30.
de Lima Cavalcanti, T. Y., Pereira, M. R., de Paula, S. O., & Franca, R. F. (2022). A review on chikungunya virus epidemiology, pathogenesis and current vaccine development. Viruses, 14(5), 969.
Yin, P., Davenport, B. J., Wan, J. J., Kim, A. S., Diamond, M. S., Ware, B. C., Tong, K., Couderc, T., Lecuit., & Kielian, M. (2023b). Chikungunya virus cell-to-cell transmission is mediated by intercellular extensions in vitro and in vivo. Nature Microbiology, 1–15
Horwood, P., & Buchy, P. (2015). Chikungunya. Revue scientifique et technique (International Office of Epizootics), 34(2), 479–489.
Silva, L. A., & Dermody, T. S. (2017). Chikungunya virus: Epidemiology, replication, disease mechanisms, and prospective intervention strategies. The Journal of Clinical Investigation, 127(3), 737–749.
Long, F., Fong, R. H., Austin, S. K., Chen, Z., Klose, T., Fokine, A., Liu, Y., Porta, J., Sapparapu, G., Akahata, W., Doranz, B. J., Crowe, J. E., Diamond, M. S., & Rossmann, M. G. (2015). Cryo-EM structures elucidate neutralizing mechanisms of anti-chikungunya human monoclonal antibodies with therapeutic activity. Proceedings of the National Academy of Sciences, 112(45), 13898–13903.
Basurko, C., Hcini, N., Demar, M., Abboud, P., Nacher, M., Carles, G., Lambert, V., Matheus, S., Group, C. S. (2022). Symptomatic Chikungunya virus infection and pregnancy outcomes: A nested case-control study in French Guiana. Viruses, 14(12), 2705.
Martins, E. B., Quintana, M. S., Silva, M. F., de Bruycker-Nogueira, F., Moraes, I. C., Rodrigues, C. D., Santos, C. C., Sampaio, S. A., Pina-Costa, A., Fabri, A. A., & Guerra-Campos, V. (2023). Predictors of chronic joint pain after Chikungunya virus infection in the INOVACHIK prospective cohort study. Journal of Clinical Virology, 169, 105610.
Martins, E. B., Silva, M. F., Tassinari, W. S., de Bruycker-Nogueira, F., Moraes, I. C., Rodrigues, C. D., Santos, C. C., Sampaio, S. A., Pina-Costa, A., Fabri, A. A., & Guerra-Campos, V. (2022). Detection of Chikungunya virus in bodily fluids: The INOVACHIK cohort study. PLoS Neglected Tropical Diseases, 16(3), e0010242.
Tun, Y. M., Charunwatthana, P., Duangdee, C., Satayarak, J., Suthisawat, S., Likhit, O., Lakhotia, D., Kosoltanapiwat, N., Sukphopetch, P., & Boonnak, K. (2022). Virological, serological and clinical analysis of Chikungunya virus infection in Thai patients. Viruses, 14(8), 1805.
Bhatnagar, S., Kumar, P., Mohan, T., Verma, P., Parida, M., Hoti, S., & Rao, D. (2015). Evaluation of multiple antigenic peptides based on the Chikungunya E2 protein for improved serological diagnosis of infection. Viral Immunology, 28(2), 107–112.
Jain, J., Okabayashi, T., Kaur, N., Nakayama, E., Shioda, T., Gaind, R., Kurosu, T., & Sunil, S. (2018). Evaluation of an immunochromatography rapid diagnosis kit for detection of chikungunya virus antigen in India, a dengue-endemic country. Virology Journal, 15(1), 1–6.
George, A., Amrutha, M., Srivastava, P., Sai, V., Sunil, S., & Srinivasan, R. (2019). Label-free detection of chikungunya non-structural protein 3 using electrochemical impedance spectroscopy. Journal of The Electrochemical Society, 166(14), B1356.
Campos, E. V. R., Luiz, J., de Oliveira, D., Abrantes, C., Rogério, C. B., Bueno, C., Miranda, V. R., Monteiro, R. A., & Fraceto, L. F. (2020). Recent developments in nanotechnology for detection and control of aedes aegypti-borne diseases. Front Bioeng Biotechnol, 8, 102.
Gowdhami, D., & Balaji, V. (2023). Design and analysis of a photonic crystal-based biosensor for the detection of chikungunya virus. Laser Physics, 33(8), 085602.
Sharma, A., Mishra, R. K., Yugender Goud, K., Mohamed, M. A., Kummari, S., Tiwari, S., Li, Z., Narayan, R., Stanciu, L. A., & Marty, J. L. (2021). Optical biosensors for diagnostics of infectious viral disease: A recent update. Diagnostics, 11(11), 2083.
Sharma, S., Kumar, A., Singh, K. S., & Tyagi, H. K. (2021). 2D photonic crystal based biosensor for the detection of chikungunya virus. Optik, 237, 166575.
Moradi, N., Taghizadeh, S. M., Hadi, N., Ghanbariasad, A., Berenjian, A., Khoo, K. S., Varjani, S., Show, P. L., & Ebrahiminezhad, A. (2022). Synthesis of mesoporous antimicrobial herbal nanomaterial-carrier for silver nanoparticles and antimicrobial sensing. Food and Chemical Toxicology, 165, 113077.
Karim, S. S., Murtaza, Z., Farrukh, S., Umer, M. A., Ali, S. S., Younas, M., Mubashir, M., Saqib, S., Ayoub, M., Bokhari, A., & Peter, A. P. (2022). Future advances and challenges of nanomaterial-based technologies for electromagnetic interference-based technologies: A review. Environmental research, 205, 112402.
Khoo, K. S., Chia, W. Y., Tang, D. Y. Y., Show, P. L., Chew, K. W., & Chen, W.-H. (2020). Nanomaterials utilization in biomass for biofuel and bioenergy production. Energies, 13(4), 892.
Varier, K. M., Gudeppu, M., Chinnasamy, A., Thangarajan, S., Balasubramanian, J., Li, Y., & Gajendran, B. (2019). Nanoparticles: antimicrobial applications and its prospects. Advanced nanostructured materials for environmental remediation (pp. 321–355). Cham: Springer.
Faghihkhorasani, A., Ahmed, H. H., Mashool, N. M., Alwan, M., Assefi, M., Adab, A. H., Yasamineh, S., Gholizadeh, O., & Baghani, M. (2023). The potential use of bacteria and bacterial derivatives as drug delivery systems for viral infection. Virology Journal, 20(1), 222.
Lau, Z. L., Low, S. S., Ezeigwe, E. R., Chew, K. W., Chai, W. S., Bhatnagar, A., Yap, Y. J., & Show, P. L. (2022). A review on the diverse interactions between microalgae and nanomaterials: Growth variation, photosynthetic performance and toxicity. Bioresource Technology, 351, 127048.
Nasiri, K., Masoumi, S. M., Amini, S., Goudarzi, M., Tafreshi, S. M., Bagheri, A., Yasamineh, S., Alwan, M., Arellano, M. T. C., & Gholizadeh, O. (2023). Recent advances in metal nanoparticles to treat periodontitis. Journal of Nanobiotechnology, 21(1), 283.
Norouzi, M., Yasamineh, S., Montazeri, M., Dadashpour, M., Sheervalilou, R., Abasi, M., & Pilehvar-Soltanahmadi, Y. (2019). Recent advances on nanomaterials-based fluorimetric approaches for microRNAs detection. Materials Science and Engineering: C, 104, 110007.
Low, S. S., Ji, D., Chai, W. S., Liu, J., Khoo, K. S., Salmanpour, S., Karimi, F., Deepanraj, B., & Show, P. L. (2021). Recent progress in nanomaterials modified electrochemical biosensors for the detection of MicroRNA. Micromachines, 12(11), 1409.
Low, S. S., Lim, C. N., Yew, M., Chai, W. S., Low, L. E., Manickam, S., Tey, B. T., & Show, P. L. (2021). Recent ultrasound advancements for the manipulation of nanobiomaterials and nanoformulations for drug delivery. Ultrasonics sonochemistry, 80, 105805.
Gholizadeh, O., Akbarzadeh, S., Moein, M., Yasamineh, S., Hosseini, P., Afkhami, H., Amini, P., Dadashpour, M., Tahavvori, A., Eslami, M., & hossein Taherian, M. (2023). The role of non-coding RNAs in the diagnosis of different stages (HCC, CHB, OBI) of hepatitis B infection. Microbial Pathogenesis. https://doi.org/10.1016/j.micpath.2023.105995
Hu, J., Stojanović, J., Yasamineh, S., Yasamineh, P., Karuppannan, S. K., Hussain Dowlath, M. J., & Serati-Nouri, H. (2021). The potential use of microRNAs as a therapeutic strategy for SARS-CoV-2 infection. Archives of Virology, 166, 2649–2672.
Oveili, E., Vafaei, S., Bazavar, H., Eslami, Y., Mamaghanizadeh, E., Yasamineh, S., & Gholizadeh, O. (2023). The potential use of mesenchymal stem cells-derived exosomes as microRNAs delivery systems in different diseases. Cell Communication and Signaling, 21(1), 1–26.
Roy, E., & Byrareddy, S. N. (2020). Role of MicroRNAs in bone pathology during Chikungunya virus infection. Viruses. https://doi.org/10.3390/v12111207
Kang, J., Tahir, A., Wang, H., & Chang, J. (2021). Applications of nanotechnology in virus detection, tracking, and infection mechanisms. WIREs Nanomedicine and Nanobiotechnology, 13(4), e1700. https://doi.org/10.1002/wnan.1700
Singhal, C., Dubey, A., Mathur, A., Pundir, C., & Narang, J. (2018). Paper based DNA biosensor for detection of chikungunya virus using gold shells coated magnetic nanocubes. Process Biochemistry, 74, 35–42.
Singhal, C., Khanuja, M., Chaudhary, N., Pundir, C., & Narang, J. (2018). Detection of chikungunya virus DNA using two-dimensional MoS2 nanosheets based disposable biosensor. Scientific Reports, 8(1), 7734.
Singhal, C., Khanuja, M., Chaudhary, N., Pundir, C., & Narang, J. (2018). Detection of chikungunya virus DNA using two-dimensional MoS2 nanosheets based disposable biosensor. Scientific Reports, 8(1), 1–11.
Singhal, C., Khanuja, M., Chaudhary, N., Pundir, C. S., & Narang, J. (2018). Detection of chikungunya virus DNA using two-dimensional MoS(2) nanosheets based disposable biosensor. Science and Reports, 8(1), 7734. https://doi.org/10.1038/s41598-018-25824-8
Singh, T. I., Singh, P., & Karki, B. (2023). Early detection of chikungunya virus utilizing the surface plasmon resonance comprising a silver-silicon-PtSe2 multilayer structure. Plasmonics. https://doi.org/10.1007/s11468-023-01840-x
Sharma, P., Hassan, H., Hasan, M. R., Fatima, T., Mohan, H., Khanuja, M., Kaushik, S., & Narang, J. (2023). PBIS-based system integrated with zinc–silver nanocomposite for the detection of Chikungunya virus. Biosensors and Bioelectronics: X, 13, 100303.
Khan, M., Hasan, M., Hossain, S., Ahommed, M., & Daizy, M. (2020). Ultrasensitive detection of pathogenic viruses with electrochemical biosensor: State of the art. Biosensors and Bioelectronics, 166, 112431.
Huang, X., Zhu, Y., & Kianfar, E. (2021). Nano biosensors: Properties, applications and electrochemical techniques. Journal of Materials Research and Technology, 12, 1649–1672.
Lopez, S., Yao, J.-S., Kuhn, R. J., Strauss, E. G., & Strauss, J. H. (1994). Nucleocapsid-glycoprotein interactions required for assembly of alphaviruses. Journal of virology, 68(3), 1316–1323.
Shirako, Y., Strauss, E. G., & Strauss, J. H. (2000). Suppressor mutations that allow Sindbis virus RNA polymerase to function with nonaromatic amino acids at the N-terminus: Evidence for interaction between nsP1 and nsP4 in minus-strand RNA synthesis. Virology, 276(1), 148–160.
Yin, P., Davenport, B. J., Wan, J. J., Kim, A. S., Diamond, M. S., Ware, B. C., Tong, K., Couderc, T., Lecuit, M., Lai, J. R., Morrison, T. E., & Kielian, M. (2023). Chikungunya virus cell-to-cell transmission is mediated by intercellular extensions in vitro and in vivo. Nature Microbiology, 8(9), 1653–1667.
Kendall, C., Khalid, H., Müller, M., Banda, D. H., Kohl, A., Merits, A., Stonehouse, N. J., & Tuplin, A. (2019). Structural and phenotypic analysis of Chikungunya virus RNA replication elements. Nucleic Acids Research, 47(17), 9296–9312.
Khan, A. H., Morita, K., del Carmen Parquet, M., Hasebe, F., Mathenge, E. G., & Igarashi, A. (2002). Complete nucleotide sequence of chikungunya virus and evidence for an internal polyadenylation site. Journal of General Virology, 83(12), 3075–3084.
Khongwichit, S., Chansaenroj, J., Chirathaworn, C., & Poovorawan, Y. (2021). Chikungunya virus infection: Molecular biology, clinical characteristics, and epidemiology in Asian countries. Journal of Biomedical Science, 28(1), 1–17.
Schmaljohn, A. L., & McClain, D. (1996). Alphaviruses (togaviridae) and flaviviruses (flaviviridae). Medical Microbiology. 4th edition.
Vasiljeva, L., Merits, A., Auvinen, P., & Kääriäinen, L. (2000). Identification of a Novel function of the alphaviruscapping apparatus: RNA 5′-triphosphatase activity of Nsp2. Journal of Biological Chemistry, 275(23), 17281–17287.
Strauss, E. G., & Strauss, J. H. (1986). Structure and replication of the alphavirus genome. The Togaviridae and Flaviviridae (pp. 35–90). Cham: Springer.
Powers, A. M., Brault, A. C., Tesh, R. B., & Weaver, S. C. (2000). Re-emergence of Chikungunya and O’nyong-nyong viruses: Evidence for distinct geographical lineages and distant evolutionary relationships. Journal of General Virology, 81(2), 471–479.
Carrasquilla, C. T., Henry, A., & Navarro, J. C. (2012). 19. Leparc-Goffart I., Nougairede A., Cassadou S., Prat C., de Lamballerie X. Chikungunya in the Americas.//Lancet. 2014; 383: 514. doi: 10.1016/S0140-6736 (14): 60185-89. 20. Likos A., Griffin I., Bingham AM, Stanek D., Fischer. Journal of Internal Medicine, 23(4), 325–329.
Lanciotti, R. S., & Valadere, A. M. (2014). Transcontinental movement of Asian genotype chikungunya virus. Emerging Infectious Diseases, 20(8), 1400.
Powers, A. M. (2016). How Chikungunya virus virology affects its epidemiology and transmission: implications for influencing public health. The Journal of Infectious Diseases., 214(suppl_5), S449–S452.
Yergolkar, P., Tandale, B., Arankalle, V., Sathe, P., Gandhe, S., Gokhle, M., Jacob, G., Hundekar, S., Mishra, A., & Sudeep, A. B. (2006). Chikungunya outbreaks caused by African genotype, India. Emerging Infectious Diseases, 12(10), 1580.
Ge, N., Sun, J., Liu, Z., Shu, J., Yan, H., Zhihua Kou, Yu., & Wei, X. J. (2022). An mRNA vaccine encoding Chikungunya virus E2–E1 protein elicits robust neutralizing antibody responses and CTL immune responses. Virologica Sinica. https://doi.org/10.1016/j.virs.2022.01.032
Natrajan, M. S., Rojas, A., & Waggoner, J. J. (2019). Beyond fever and pain: Diagnostic methods for chikungunya virus. Journal of Clinical Microbiology, 57(6), e00350-e1319.
Hussien, F. H. (2023). An overview of Chikungunya disease, origins, symptoms, transmission, route of infection, diagnosis, and treatment. Medical Journal of Babylon, 20(2), 240–243.
Bartholomeeusen, K., Daniel, M., LaBeaud, D. A., Gasque, P., Peeling, R. W., Stephenson, K. E., Ng, L. F. P., & Ariën, K. K. (2023). Chikungunya fever. Nature Reviews Disease Primers, 9(1), 17.
Calabrese, L. H. (2008). Emerging viral infections and arthritis: The role of the rheumatologist. Nature clinical practice Rheumatology, 4(1), 2–3.
Dupuis-Maguiraga, L., Noret, M., Brun, S., Le Grand, R., Gras, G., & Roques, P. (2012). Chikungunya disease: Infection-associated markers from the acute to the chronic phase of arbovirus-induced arthralgia. PLoS Neglected Tropical Diseases, 6(3), e1446.
Hoarau, J.-J., Bandjee, M.-C., Trotot, P. K., Das, T., Li-Pat-Yuen, G., Dassa, B., Denizot, M., Guichard, E., Ribera, A., Henni, T., Tallet, F., Moiton, M. P., Gauzère, B. A., Bruniquet, S., Bandjee, Z. J., Morbidelli, P., Martigny, G., Jolivet, M., Gay, F., … Gasque, P. (2010). Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. The Journal of Immunology, 184(10), 5914–5927.
Phuklia, W., Kasisith, J., Modhiran, N., Rodpai, E., Thannagith, M., Thongsakulprasert, T., Smith, D. R., & Ubol, S. (2013). Osteoclastogenesis induced by CHIKV-infected fibroblast-like synoviocytes: a possible interplay between synoviocytes and monocytes/macrophages in CHIKV-induced arthralgia/arthritis. Virus Research, 177(2), 179–188.
Teo, T.-H., Lum, F.-M., Claser, C., Lulla, V., Lulla, A., Merits, A., Rénia, L., & Ng, L. F. P. (2013). A pathogenic role for CD4+ T cells during Chikungunya virus infection in mice. The Journal of Immunology, 190(1), 259–269.
Chow, A., Her, Z., Ong, E. K. S., Chen, J.-M., Dimatatac, F., Kwek, D. J. C., Barkham, T., Yang, H., Rénia, L., Leo, Y.-S., & Ng, L. F. P. (2011). Persistent arthralgia induced by Chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. Journal of Infectious Diseases, 203(2), 149–157.
Abdelnabi, R., Neyts, J., & Delang, L. (2016). Antiviral strategies against Chikungunya virus. Methods in Molecular Biology (Clifton, N.J.), 1426, 243–253. https://doi.org/10.1007/978-1-4939-3618-2_22.
Bouma, E. M., van de Pol, D. P., Sanders, I. D., Rodenhuis-Zybert, I. A., & Smit, J. M. (2020). Serotonergic drugs inhibit chikungunya virus infection at different stages of the cell entry pathway. Journal of Virology. https://doi.org/10.1128/jvi.00274-00220
Subudhi, B. B., Chattopadhyay, S., Mishra, P., & Kumar, A. (2018). Current strategies for inhibition of Chikungunya infection. Viruses, 10(5), 235.
Abdelnabi, R., Neyts, J., & Delang, L. (2015). Towards antivirals against chikungunya virus. Antiviral Research, 121, 59–68.
Ghildiyal, R., & Gabrani, R. (2020). Antiviral therapeutics for chikungunya virus. Expert Opinion on Therapeutic Patents, 30(6), 467–480.
Perumal, V., & Hashim, U. (2014). Advances in biosensors: Principle, architecture and applications. Journal of Applied Biomedicine, 12(1), 1–15.
Lowe, C. (1989). Biosensors. Philosophical Transactions of the Royal Society of London B, Biological Sciences, 324(1224), 487–496.
Allouzi, M. M. A., Allouzi, S., Al-Salaheen, B., Khoo, K. S., Rajendran, S., Sankaran, R., Sy-Toan, N., & Show, P. L. (2022). Current advances and future trend of nanotechnology as microalgae-based biosensor. Biochemical Engineering Journal, 187, 108653.
Mehrvar, M., & Abdi, M. (2004). Recent developments, characteristics, and potential applications of electrochemical biosensors. Analytical Sciences, 20(8), 1113–1126.
Stark, P. M., Azar, S. R., Debboun, M., Vela, J., Roundy, C. M., Rossi, S. L., Reyna, M., Hanley, K. A., Ribeiro, G. S., Kitron, U., Yun, R., Huang, J. H., Fernandez-Salas, I., Leal, G., Vasilakis, N., Weaver, S. C., Vitek, C. J., & Paploski, I. A. D. (2017). Differential vector competency of Aedes albopictus populations from the Americas for Zika virus. The American Journal of Tropical Medicine and Hygiene, 97(2), 330.
Diallo, D., Sall, A. A., Buenemann, M., Chen, R., Faye, O., Diagne, C. T., Faye, O., Ba, Y., Dia, I., Watts, D., & Weaver, S. C. (2012). Landscape ecology of sylvatic chikungunya virus and mosquito vectors in southeastern Senegal. PLoS Neglected Tropical Diseases, 6(6), e1649.
Wasik, D., Mulchandani, A., & Yates, M. V. (2018). Point-of-use nanobiosensor for detection of dengue virus NS1 antigen in adult Aedes aegypti: A potential tool for improved dengue surveillance. Analytical Chemistry, 90(1), 679–684.
Pedraza-Escalona, M., Guzmán-Bringas, O., Arrieta-Oliva, H. I., Gómez-Castellano, K., Salinas-Trujano, J., Torres-Flores, J., Muñoz-Herrera, J. C., Camacho-Sandoval, R., Contreras-Pineda, P., Chacón-Salinas, R., & Pérez-Tapia, S. M. (2021). Isolation and characterization of high affinity and highly stable anti-Chikungunya virus antibodies using ALTHEA Gold Libraries™. BMC Infectious Diseases, 21(1), 1–11.
Singh, A., Kumar, A., Yadav, R., Uversky, V. N., & Giri, R. (2018). Deciphering the dark proteome of Chikungunya virus. Scientific Reports, 8(1), 1–10.
Cajigas, S., Alzate, D., & Orozco, J. (2020). Gold nanoparticle/DNA-based nanobioconjugate for electrochemical detection of Zika virus. Microchimica Acta, 187, 1–10.
Steinmetz, M., Lima, D., Viana, A. G., Fujiwara, S. T., Pessôa, C. A., Etto, R. M., & Wohnrath, K. (2019). A sensitive label-free impedimetric DNA biosensor based on silsesquioxane-functionalized gold nanoparticles for Zika virus detection. Biosensors and Bioelectronics, 141, 111351.
Afsahi, S., Lerner, M. B., Goldstein, J. M., Lee, J., Tang, X., Bagarozzi, D. A., Pan, D., Locascio, L., Walker, A., Barron, F., & Goldsmith, B. R. (2018). Novel graphene-based biosensor for early detection of Zika virus infection. Biosensors and Bioelectronics, 100, 85–88.
Tripathi, M. N., Jangir, P., Rai, S., Gangwar, M., Nath, G., Saxena, P. S., & Srivastava, A. (2023). A novel approach for rapid and sensitive detection of Zika virus utilizing silver Nanoislands as SERS platform. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 302, 123045.
El Muttaqien, S., Khoris, I. M., Widayanti, T., Pambudi, S., & Park, E. Y. (2023). Simple, versatile, and practical impedimetric immunosensor based on gold nanoparticle-polyaniline nanocomposite for clinical dengue virus detection. Biochemical Engineering Journal, 198, 109028.
Duyen, V. T. C., Van Toi, V., Van Hoi, T., & Truong, P. L. (2023). A novel colorimetric biosensor for rapid detection of dengue virus upon acid-induced aggregation of colloidal gold. Analytical Methods, 15(32), 3991–3999.
Palomar, Q., Gondran, C., Marks, R., Cosnier, S., & Holzinger, M. (2018). Impedimetric quantification of anti-dengue antibodies using functional carbon nanotube deposits validated with blood plasma assays. Electrochimica Acta, 274, 84–90.
Solanki, S., Soni, A., Pandey, M., Biradar, A., & Sumana, G. (2018). Langmuir-Blodgett nanoassemblies of the MoS2–Au composite at the air–water interface for dengue detection. ACS Applied Materials & Interfaces, 10(3), 3020–3028.
Odeh, A. A., Al-Douri, Y., Voon, C. H., Mat Ayub, R., Gopinath, S. C. B., Odeh, R. A., Ameri, M., & Bouhemadou, A. (2017). A needle-like Cu2CdSnS4 alloy nanostructure-based integrated electrochemical biosensor for detecting the DNA of Dengue serotype 2. Microchimica Acta, 184(7), 2211–2218.
Peh, A. E. K., & Li, S. F. Y. (2013). Dengue virus detection using impedance measured across nanoporous aluminamembrane. Biosensors and Bioelectronics, 42, 391–396.
Martinez-Liu, C., Machain-Williams, C., Martinez-Acuña, N., Lozano-Sepulveda, S., Galan-Huerta, K., Arellanos-Soto, D., Meléndez-Villanueva, M., Ávalos-Nolazco, D., Pérez-Ibarra, K., Galindo-Rodríguez, S., & de Jesús Garza-Juarez, A. (2022). Development of a rapid gold nanoparticle-based lateral flow immunoassay for the detection of Dengue virus. Biosensors, 12(7), 495.
Deng, J., & Toh, C.-S. (2013). Impedimetric DNA biosensor based on a nanoporous alumina membrane for the detection of the specific oligonucleotide sequence of dengue virus. Sensors, 13(6), 7774–7785.
Anusha, J., Kim, B. C., Yu, K.-H., & Raj, C. J. (2019). Electrochemical biosensing of mosquito-borne viral disease, dengue: A review. Biosensors and Bioelectronics, 142, 111511.
Nosrati, R., Golichenari, B., Nezami, A., Taghdisi, S. M., Karimi, B., Ramezani, M., Abnous, K., & Shaegh, S. A. M. (2017). Helicobacter pylori point-of-care diagnosis: Nano-scale biosensors and microfluidic systems. TrAC Trends in Analytical Chemistry, 97, 428–444.
Pumera, M., Sanchez, S., Ichinose, I., & Tang, J. (2007). Electrochemical nanobiosensors. Sensors and Actuators B: Chemical, 123(2), 1195–1205.
Nasrin, F., Chowdhury, A. D., Ganganboina, A. B., Achadu, O. J., Hossain, F., Yamazaki, M., & Park, E. Y. (2020). Fluorescent and electrochemical dual-mode detection of Chikungunya virus E1 protein using fluorophore-embedded and redox probe-encapsulated liposomes. Microchimica Acta, 187, 1–11.
Misra, R., Acharya, S., & Sushmitha, N. (2022). Nanobiosensor-based diagnostic tools in viral infections: Special emphasis on Covid-19. Reviews in Medical Virology, 32(2), e2267. https://doi.org/10.1002/rmv.2267
Ao, H., Qian, Z., Zhu, Y., Zhao, M., Tang, C., Huang, Y., Feng, H., & Wang, A. (2016). A fluorometric biosensor based on functional Au/Ag nanoclusters for real-time monitoring of tyrosinase activity. Biosensors and Bioelectronics, 86, 542–547.
Nasrin, F., Chowdhury, A. D., Ganganboina, A. B., Achadu, O. J., Hossain, F., Yamazaki, M., & Park, E. Y. (2020). Fluorescent and electrochemical dual-mode detection of Chikungunya virus E1 protein using fluorophore-embedded and redox probe-encapsulated liposomes. Microchimica Acta, 187(12), 1–11.
Nasrin, F., Chowdhury, A. D., Takemura, K., Kozaki, I., Honda, H., Adegoke, O., & Park, E. Y. (2020). Fluorometric virus detection platform using quantum dots-gold nanocomposites optimizing the linker length variation. Analytica Chimica Acta, 1109, 148–157.
Pasinszki, T., & Krebsz, M. (2019). Advances in celiac disease testing. Advances in Clinical Chemistry, 91, 1–29.
Benito-Pena, E., Valdés, M. G., Glahn-Martinez, B., & Moreno-Bondi, M. C. (2016). Fluorescence based fiber optic and planar waveguide biosensors. A review. Analytica chimica acta, 943, 17–40.
Chen, C., & Wang, J. (2020). Optical biosensors: An exhaustive and comprehensive review. The Analyst, 145(5), 1605–1628.
George, A., Amrutha, M., Srivastava, P., Sunil, S., Sai, V., & Srinivasan, R. (2021). Development of a U-bent plastic optical fiber biosensor with plasmonic labels for the detection of chikungunya non-structural protein 3. The Analyst, 146(1), 244–252.
Camara, A. R., Gouvêa, P. M., Dias, A. C. M., Braga, A. M., Dutra, R. F., de Araujo, R. E., & Carvalho, I. C. (2013). Dengue immunoassay with an LSPR fiber optic sensor. Optics Express, 21(22), 27023–27031.
Zhang, J., & Zhao, J. (2019). Immuno-biosensor. Nano-inspired biosensors for protein assay with clinical applications (pp. 115–137). Elsevier.
Figueiredo, A., Vieira, N. C., Dos Santos, J. F., Janegitz, B. C., Aoki, S. M., Junior, P. P., Lovato, R. L., Nogueira, M. L., Zucolotto, V., & Guimaraes, F. E. (2015). Electrical detection of dengue biomarker using egg yolk immunoglobulin as the biological recognition element. Scientific Reports, 5(1), 1–5. https://europepmc.org/article/pmc/4297984
Sinawang, P. D., Rai, V., Ionescu, R. E., & Marks, R. S. (2016). Electrochemical lateral flow immunosensor for detection and quantification of dengue NS1 protein. Biosensors and Bioelectronics, 77, 400–408.
Tai, D.-F., Lin, C.-Y., Wu, T.-Z., & Chen, L.-K. (2005). Recognition of dengue virus protein using epitope-mediated molecularly imprinted film. Analytical Chemistry, 77(16), 5140–5143.
Antunes, P., Watterson, D., Parmvi, M., Burger, R., Boisen, A., Young, P., Cooper, M. A., Hansen, M. F., Ranzoni, A., & Donolato, M. (2015). Quantification of NS1 dengue biomarker in serum via optomagnetic nanocluster detection. Scientific Reports, 5(1), 1–10.
Dias, A. C. M., Gomes-Filho, S. L., Silva, M. M., & Dutra, R. F. (2013). A sensor tip based on carbon nanotube-ink printed electrode for the dengue virus NS1 protein. Biosensors and Bioelectronics, 44, 216–221.
Park, G., Kim, H.-O., Lim, J.-W., Park, C., Yeom, M., Song, D., & Haam, S. (2022). Rapid detection of influenza A (H1N1) virus by conductive polymer-based nanoparticle via optical response to virus-specific binding. Nano Research, 15(3), 2254–2262.
Atias, D., Liebes, Y., Chalifa-Caspi, V., Bremand, L., Lobel, L., Marks, R. S., & Dussart, P. (2009). Chemiluminescent optical fiber immunosensor for the detection of IgM antibody to dengue virus in humans. Sensors and actuators B: Chemical, 140(1), 206–215.
Lidya, B., Santoso, B., Yusuf, M., Alisyahbana, B., & Subroto, T. (2018). Application of malva nut gum as bioreductor and stabilizer on gold nanoparticle synthesis: Preparation of Chikungunya immunoassay biosensor. Asian Journal of Medicine and Biomedicine, 8.
Mardekian, S. K., & Roberts, A. L. (2015). Diagnostic options and challenges for dengue and chikungunya viruses. BioMed Research International. https://doi.org/10.1155/2015/834371
Nasrin, F., Tsuruga, K., Utomo, D. I. S., Chowdhury, A. D., & Park, E. Y. (2021). Design and analysis of a single system of impedimetric biosensors for the detection of mosquito-borne viruses. Biosensors, 11(10), 376.
Saraf, N., Villegas, M., Willenberg, B. J., & Seal, S. (2019). Multiplex viral detection platform based on a aptamers-integrated microfluidic channel. ACS Omega, 4(1), 2234–2240.
Sharma, S., Pardasani, D., Dash, P. K., Parida, M., & Dubey, D. K. (2020). Development of magnetic bead based sample extraction coupled polymerase spiral reaction for rapid on-site detection of Chikungunya virus. Scientific Reports, 10(1), 1–8.
Sperling, R. A., Gil, P. R., Zhang, F., Zanella, M., & Parak, W. J. (2008). Biological applications of gold nanoparticles. Chemical Society Reviews, 37(9), 1896–1908.
Carter, J. R., Balaraman, V., Kucharski, C. A., Fraser, T. S., & Fraser, M. J. (2013). A novel dengue virus detection method that couples DNAzyme and gold nanoparticle approaches. Virology Journal, 10(1), 1–15.
Burdușel, A.-C., Gherasim, O., Grumezescu, A. M., Mogoantă, L., Ficai, A., & Andronescu, E. (2018). Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials, 8(9), 681.
Kaushik, S., Sharma, V., Chhikara, S., Yadav, J., & Kaushik, S. (2019). Anti-chikungunya activity of green synthesized silver nanoparticles using Carica papaya leaves in animal cell culture model. Asian Journal of Pharmaceutical and Clinical Research, 12(6), 170–174.
Yupapin, P., Trabelsi, Y., Vigneswaran, D., Taya, S. A., Daher, M. G., & Colak, I. (2022a). Ultra-high-sensitive sensor based on surface plasmon resonance structure having Si and graphene layers for the detection of chikungunya virus. Plasmonics, 1–7
Díaz-González, M., de la Escosura-Muñiz, A., Fernandez-Argüelles, M. T., Alonso, F. J. G., & Costa-Fernandez, J. M. (2020). Quantum dot bioconjugates for diagnostic applications. Surface-modified nanobiomaterials for electrochemical and biomedicine applications (pp. 133–176). Springer.
Jalal, N. R., Mehrbod, P., Shojaei, S., Labouta, H. I., Mokarram, P., Afkhami, A., Madrakian, T., Los, M. J., Schaafsma, D., Giersig, M., Ahmadi, M., & Ghavami, S. (2021). Magnetic nanomaterials in microfluidic sensors for virus detection: A review. ACS Applied Nano Materials, 4(5), 4307–4328.
Nguyen, M. D., Tran, H.-V., Xu, S., & Lee, T. R. (2021). Fe3O4 Nanoparticles: Structures, synthesis, magnetic properties, surface functionalization, and emerging applications. Applied Sciences, 11(23), 11301.
Ghazanfari, M. R., Kashefi, M., Shams, S. F., & Jaafari, M. R. (2016). Perspective of Fe3O4 nanoparticles role in biomedical applications. Biochemistry Research International. https://doi.org/10.1155/2016/7840161
Peng, D., Yang, Y., Que, M., Ding, Y., Wu, M., Deng, X., He, Q., Ma, X., Li, X., & Qiu, H. (2023). Partially oxidized MoS2 nanosheets with high water-solubility to enhance the peroxidase-mimic activity for sensitive detection of glutathione. Analytica Chimica Acta, 1250, 340968.
Xia, C., Xu, L., Li, Z., & Guo, L. (2023). Sulfur defect-engineered MoS2 nanosheets with enhanced peroxidase-like activity. Applied Surface Science, 635, 157777.
Huang, K.-J., Liu, Y.-J., Wang, H.-B., Wang, Y.-Y., & Liu, Y.-M. (2014). Sub-femtomolar DNA detection based on layered molybdenum disulfide/multi-walled carbon nanotube composites, Au nanoparticle and enzyme multiple signal amplification. Biosensors and Bioelectronics, 55, 195–202.
Yan, L., Shi, H., Sui, X., Deng, Z., & Gao, L. (2017). MoS 2-DNA and MoS 2 based sensors. Rsc Advances, 7(38), 23573–23582.
Shukla, S., Grover, N., & Arora, P. (2023). Resolution enhancement using a multi-layered Aluminum-based plasmonic device for chikungunya virus detection. Optical and Quantum Electronics, 55(3), 274.
Yupapin, P., Trabelsi, Y., Vigneswaran, D., Taya, S. A., Daher, M. G., & Colak, I. (2022). Ultra-high-sensitive sensor based on surface plasmon resonance structure having Si and graphene layers for the detection of chikungunya virus. Plasmonics, 17(3), 1315–1321.
Van Tuan, D., Ngan, D. T. T., Thuy, N. T., Lan, H., Nguyet, N. T., Van Thu, Vu., Hung, V.-P., & Tam, P. D. (2020). Effect of nanostructured MoS2 morphology on the glucose sensing of electrochemical biosensors. Current Applied Physics, 20(9), 1090–1096.
Daher, M. G., Alsalman, O., Ahmed, N. M., Sassi, I., Sorathiya, V., Tsui, H. C. L., & Patel, S. K. (2023). Modeling of a novel chikungunya virus detector based on silicon and titanium nitride multilayer thin films. Optik, 287, 171136.
Jain, J., Nayak, K., Tanwar, N., Gaind, R., Bhupendra Gupta, J. S., Shastri, R. K., Bhatnagar, M. K., Kaja, A. C., & Sunil, S. (2017). Clinical, serological, and virological analysis of 572 chikungunya patients from 2010 to 2013 in India. Clinical Infectious Diseases, 65(1), 133–140.
Syahir, A., Usui, K., Tomizaki, K.-Y., Kajikawa, K., & Mihara, H. (2015). Label and label-free detection techniques for protein microarrays. Microarrays, 4(2), 228–244.
Falina, S., Anuar, K., Shafiee, S. A., Juan, J. C., Manaf, A. A., Kawarada, H., & Syamsul, M. (2022). Two-dimensional non-carbon materials-based electrochemical printed sensors: An updated review. Sensors, 22(23), 9358.
Wang, J., Drelich, A. J., Hopkins, C. M., & Mecozzi, S. (2022). Gold nanoparticles in virus detection: Recent advances and potential considerations for SARS-CoV-2 testing development. WIREs Nanomedicine and Nanobiotechnology, 14(1), e1754. https://doi.org/10.1002/wnan.1754
Draz, M. S., & Shafiee, H. (2018). Applications of gold nanoparticles in virus detection. Theranostics, 8(7), 1985–2017. https://doi.org/10.7150/thno.23856
Hu, X., Zhang, Y., Ding, T., Liu, J., & Zhao, H. (2020). Multifunctional gold nanoparticles: A novel nanomaterial for various medical applications and biological activities. Front Bioeng Biotechnol, 8, 990.
Kumar, C. S., Hormes, J., & Leuschner, C. (2006). Nanofabrication towards biomedical applications: Techniques, tools, applications, and impact. Wiley.
Xu, L., Wang, Y. Y., Huang, J., Chen, C. Y., Wang, Z. X., & Xie, H. (2020). Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics, 10(20), 8996–9031. https://doi.org/10.7150/thno.45413
Ghosh, U., Sayef Ahammed, K., Mishra, S., & Bhaumik, A. (2022). The emerging roles of silver nanoparticles to target viral life cycle and detect viral pathogens. Chemistry–An Asian Journal, 17(5), e202101149.
Deroco, P. B., Junior, D. W., & Kubota, L. T. (2021). Recent advances in point-of-care biosensors for the diagnosis of neglected tropical diseases. Sensors and Actuators B: Chemical, 349, 130821.
Pirzada, M., & Altintas, Z. (2022). Nanomaterials for virus sensing and tracking. Chemical Society Reviews, 51(14), 5805–5841. https://pubs.rsc.org/en/content/articlelanding/2022/cs/d1cs01150b
Sharma, P., Hasan, M. R., Khanuja, M., & Narang, J. (2023). Carbon ink printed flexible glove-based aptasensor for rapid and point of care detection of Chikungunya virus. Process Biochemistry, 133, 1–10. https://www.sciencedirect.com/science/article/abs/pii/S1359511323002623
Bossuyt, P. M., Reitsma, J. B., Bruns, D. E., Gatsonis, C. A., Glasziou, P. P., Irwig, L. M., Lijmer, J. G., Moher, D. R. D., & de Vet, H. C. W. (2003). Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Clinical Chemistry and Laboratory Medicine. https://doi.org/10.1515/CCLM.2003.012
Song, M., Yang, M., & Hao, J. (2021). Pathogenic virus detection by optical nanobiosensors. Cell Reports Physical Science. https://doi.org/10.1016/j.xcrp.2020.100288
Acknowledgements
Thanks to all the scientists of the world who advance science and cure pain with their efforts.
Funding
The authors received no financial support for the authorship and publication of this article.
Author information
Authors and Affiliations
Contributions
OG, SY, HA, and AK wrote the main manuscript text and SY and prepared the figures. SASH, ZS, MG, JA, MH, reviewed and editing and OG, and HZ corresponding authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors have full consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shahrtash, S.A., Ghnim, Z.S., Ghaheri, M. et al. Recent Advances in the Role of Different Nanoparticles in the Various Biosensors for the Detection of the Chikungunya Virus. Mol Biotechnol (2024). https://doi.org/10.1007/s12033-024-01052-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12033-024-01052-6