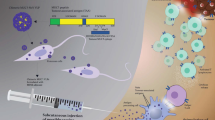
Abstract
Carbonic anhydrase IX (CAIX) is a cancer-associated membrane protein frequently overexpressed in hypoxic solid tumours leading to enhanced tumour cell survival and invasion, and it has been proposed to be an attractive tumour-specific molecule for antibody-mediated targeting. This study aimed to generate a virus-like particle (VLP)-based CAIX vaccine candidate and evaluate its efficacy in a mouse model of breast cancer. The prototype murine vaccine was developed based on the ssRNA bacteriophage Qbeta VLPs with chemically coupled murine CAIX protein catalytic domains on their surfaces. The vaccine was shown to efficiently break the natural B cell tolerance against autologous murine CAIX and to induce high-titre Th1-oriented IgG responses in the BALB/c mice. This vaccine was tested in a therapeutic setting by using a triple-negative breast cancer mouse model system comprising 4T1, 4T1-Car9KI and 4T1-Car9KO cells, the latter representing positive and negative controls for murine CAIX production, respectively. The humoural immune responses induced in tumour-bearing animals were predominantly of Th1-type and higher anti-mCAIXc titres correlated with slower growth and lung metastasis development of 4T1 tumours constitutively expressing mCAIX in vivo in the syngeneic host.






Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- CAIX:
-
Carbonic anhydrase IX
- CMV:
-
Cytomegalovirus
- H&E:
-
Haematoxylin and eosin
- IVC:
-
Individually ventilated cage
- KO:
-
Knock-out
- KI:
-
Knock-in
- mCAIXc:
-
Catalytic domain of murine carbonic anhydrase IX
- ssRNA:
-
Single-stranded RNA
- SPF:
-
Specific pathogen-free
- TAA:
-
Tumour-associated antigen
- TNBC:
-
Triple-negative breast cancer
- VLP:
-
Virus-like particle
References
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71, 209–249.
Couzin-Frankel, J. (2013). Breakthrough of the year 2013. Cancer immunotherapy. Science, 342, 1432–1433.
Antonarelli, G., Corti, C., Tarantino, P., Ascione, L., Cortes, J., Romero, P., Mittendorf, E. A., Disis, M. L., & Curigliano, G. (2021). Therapeutic cancer vaccines revamping: Technology advancements and pitfalls. Annals of Oncology, 32, 1537–1551.
Buonaguro, L., & Tagliamonte, M. (2020). Selecting target antigens for cancer vaccine development. Vaccines (Basel). https://doi.org/10.3390/vaccines8040615
Pumpens, P., Renhofa, R., Dishlers, A., Kozlovska, T., Ose, V., Pushko, P., Tars, K., Grens, E., & Bachmann, M. F. (2016). The True Story and Advantages of RNA Phage Capsids as Nanotools. Intervirology, 59, 74–110.
Jennings, G. T., & Bachmann, M. F. (2008). The coming of age of virus-like particle vaccines. Biological Chemistry, 389, 521–536.
Rumnieks, J., & Tars, K. (2018). Protein-RNA Interactions in the Single-Stranded RNA Bacteriophages. SubCellular Biochemistry, 88, 281–303.
Pickett, G. G., & Peabody, D. S. (1993). Encapsidation of heterologous RNAs by bacteriophage MS2 coat protein. Nucleic Acids Research, 21, 4621–4626.
Lieknina, I., Cernova, D., Rumnieks, J., & Tars, K. (2020). Novel ssRNA phage VLP platform for displaying foreign epitopes by genetic fusion. Vaccine, 38, 6019–6026.
Bachmann, M. F., Zeltins, A., Kalnins, G., Balke, I., Fischer, N., Rostaher, A., Tars, K., & Favrot, C. (2018). Vaccination against IL-31 for the treatment of atopic dermatitis in dogs. The Journal of Allergy and Clinical Immunology, 142(279–281), e271.
Fettelschoss-Gabriel, A., Fettelschoss, V., Olomski, F., Birkmann, K., Thoms, F., Buhler, M., Kummer, M., Zeltins, A., Kundig, T. M., & Bachmann, M. F. (2019). Active vaccination against interleukin-5 as long-term treatment for insect-bite hypersensitivity in horses. Allergy, 74, 572–582.
Tissot, A. C., Maurer, P., Nussberger, J., Sabat, R., Pfister, T., Ignatenko, S., Volk, H. D., Stocker, H., Muller, P., Jennings, G. T., Wagner, F., & Bachmann, M. F. (2008). Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: A double-blind, randomised, placebo-controlled phase IIa study. Lancet, 371, 821–827.
Cavelti-Weder, C., Timper, K., Seelig, E., Keller, C., Osranek, M., Lassing, U., Spohn, G., Maurer, P., Muller, P., Jennings, G. T., Willers, J., Saudan, P., Donath, M. Y., & Bachmann, M. F. (2016). Development of an interleukin-1beta vaccine in patients with type 2 diabetes. Molecular Therapy, 24, 1003–1012.
Chang, D. K., Moniz, R. J., Xu, Z., Sun, J., Signoretti, S., Zhu, Q., & Marasco, W. A. (2015). Human anti-CAIX antibodies mediate immune cell inhibition of renal cell carcinoma in vitro and in a humanized mouse model in vivo. Molecular Cancer, 14, 119.
Opavsky, R., Pastorekova, S., Zelnik, V., Gibadulinova, A., Stanbridge, E. J., Zavada, J., Kettmann, R., & Pastorek, J. (1996). Human MN/CA9 gene, a novel member of the carbonic anhydrase family: Structure and exon to protein domain relationships. Genomics, 33, 480–487.
Nogradi, A. (1998). The role of carbonic anhydrases in tumors. American Journal of Pathology, 153, 1–4.
van Kuijk, S. J., Yaromina, A., Houben, R., Niemans, R., Lambin, P., & Dubois, L. J. (2016). Prognostic significance of carbonic anhydrase IX expression in cancer patients: A meta-analysis. Frontiers in Oncology, 6, 69.
Pastorek, J., Pastorekova, S., Callebaut, I., Mornon, J. P., Zelnik, V., Opavsky, R., Zat’ovicova, M., Liao, S., Portetelle, D., Stanbridge, E. J., et al. (1994). Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene, 9, 2877–2888.
Queen, A., Bhutto, H. N., Yousuf, M., Syed, M. A., & Hassan, M. I. (2022). Carbonic anhydrase IX: A tumor acidification switch in heterogeneity and chemokine regulation. Seminars in Cancer Biology, 86, 899–913.
Pastorekova, S., & Gillies, R. J. (2019). The role of carbonic anhydrase IX in cancer development: Links to hypoxia, acidosis, and beyond. Cancer and Metastasis Reviews, 38, 65–77.
Singh, S., Lomelino, C. L., Mboge, M. Y., Frost, S. C., & McKenna, R. (2018). Cancer drug development of carbonic anhydrase inhibitors beyond the active site. Molecules. https://doi.org/10.3390/molecules23051045
Kciuk, M., Gielecinska, A., Mujwar, S., Mojzych, M., Marciniak, B., Drozda, R., & Kontek, R. (2022). Targeting carbonic anhydrase IX and XII isoforms with small molecule inhibitors and monoclonal antibodies. Journal of Enzyme Inhibition and Medicinal Chemistry, 37, 1278–1298.
Campos, N. S. P., Souza, B. S., Silva, G., Porto, V. A., Chalbatani, G. M., Lagreca, G., Janji, B., & Suarez, E. R. (2022). Carbonic anhydrase IX: A renewed target for cancer immunotherapy. Cancers (Basel). https://doi.org/10.3390/cancers14061392
Bleumer, I., Knuth, A., Oosterwijk, E., Hofmann, R., Varga, Z., Lamers, C., Kruit, W., Melchior, S., Mala, C., Ullrich, S., De Mulder, P., Mulders, P. F., & Beck, J. (2004). A phase II trial of chimeric monoclonal antibody G250 for advanced renal cell carcinoma patients. British Journal of Cancer, 90, 985–990.
Chamie, K., Donin, N. M., Klopfer, P., Bevan, P., Fall, B., Wilhelm, O., Storkel, S., Said, J., Gambla, M., Hawkins, R. E., Jankilevich, G., Kapoor, A., Kopyltsov, E., Staehler, M., Taari, K., Wainstein, A. J. A., Pantuck, A. J., & Belldegrun, A. S. (2017). Adjuvant weekly girentuximab following nephrectomy for high-risk renal cell carcinoma: The ARISER randomized clinical trial. JAMA Oncology, 3, 913–920.
Hua, Z., White, J., & Zhou, J. (2022). Cancer stem cells in TNBC. Seminars in Cancer Biology, 82, 26–34.
Ong, C. H. C., Lee, D. Y., Lee, B., Li, H., Lim, J. C. T., Lim, J. X., Yeong, J. P. S., Lau, H. Y., Thike, A. A., Tan, P. H., & Iqbal, J. (2022). Hypoxia-regulated carbonic anhydrase IX (CAIX) protein is an independent prognostic indicator in triple negative breast cancer. Breast Cancer Research, 24, 38.
Ivanova, L., Zandberga, E., Silina, K., Kalnina, Z., Abols, A., Endzelins, E., Vendina, I., Romanchikova, N., Hegmane, A., Trapencieris, P., Eglitis, J., & Line, A. (2015). Prognostic relevance of carbonic anhydrase IX expression is distinct in various subtypes of breast cancer and its silencing suppresses self-renewal capacity of breast cancer cells. Cancer Chemotherapy and Pharmacology, 75, 235–246.
Pastorekova, S., Parkkila, S., Parkkila, A. K., Opavsky, R., Zelnik, V., Saarnio, J., & Pastorek, J. (1997). Carbonic anhydrase IX, MN/CA IX: Analysis of stomach complementary DNA sequence and expression in human and rat alimentary tracts. Gastroenterology, 112, 398–408.
Takacova, M., Barathova, M., Zatovicova, M., Golias, T., Kajanova, I., Jelenska, L., Sedlakova, O., Svastova, E., Kopacek, J., & Pastorekova, S. (2019). Carbonic anhydrase IX-mouse versus human. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms21010246
Leitans, J., Kazaks, A., Balode, A., Ivanova, J., Zalubovskis, R., Supuran, C. T., & Tars, K. (2015). Efficient expression and crystallization system of cancer-associated carbonic anhydrase isoform IX. Journal of Medicinal Chemistry, 58, 9004–9009.
Brune, K. D., Lieknina, I., Sutov, G., Morris, A. R., Jovicevic, D., Kalnins, G., Kazaks, A., Kluga, R., Kastaljana, S., Zajakina, A., Jansons, J., Skrastina, D., Spunde, K., Cohen, A. A., Bjorkman, P. J., Morris, H. R., Suna, E., & Tars, K. (2021). N-terminal modification of Gly-his-tagged proteins with azidogluconolactone. ChemBioChem, 22, 3199–3207.
Kalniņa, Z.L., I., Koteloviča, S., Petrovska, R., Laugalis, M.T., Skeltona, V., Jansons, J., Kreishmane, M., Tārs, K. (2023) Development of 4T1 breast cancer mouse model system for preclinical carbonic anhydrase IX studies. Submitted to Heliyon.
G*Power Statistical Power Analyses for Mac and Windows. Available from: http://www.gpower.hhu.de/.
Faul, F., Erdfelder, E., Lang, A. G., & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191.
FELASA Working Group on Revision of Guidelines for Health Monitoring of Rodents and Rabbits, Mahler-Convenor, M., Berard, M., Feinstein, R., Gallagher, A., Illgen-Wilcke, B., Pritchett-Corning, K., & Raspa, M. (2014). FELASA recommendations for the health monitoring of mouse rat hamster guinea pig and rabbit colonies in breeding and experimental units. Laboratory Animals, 48, 178–192.
Workman, P., Aboagye, E. O., Balkwill, F., Balmain, A., Bruder, G., Chaplin, D. J., Double, J. A., Everitt, J., Farningham, D. A., Glennie, M. J., Kelland, L. R., Robinson, V., Stratford, I. J., Tozer, G. M., Watson, S., Wedge, S. R., & Eccles, S. A. (2010). Guidelines for the welfare and use of animals in cancer research. British Journal of Cancer, 102, 1555–1577.
Expert Working Group (Animal Welfare Body of the European Commission). (2013). Examples to illustrate the process of severity classification, day-to-day assessment and actual severity assessment. European Commission. [Cited 1 Mar 2021]. Available from: https://ec.europa.eu/environment/chemicals/lab_animals/pdf/examples.pdf.
Slaoui, M., & Fiette, L. (2011). Histopathology procedures: From tissue sampling to histopathological evaluation. Methods in Molecular Biology, 691, 69–82.
Pumpens, P., & Pushko, P. (2022). Virus-Like Particles: A Comprehensive Guide. CRC Press.
Nimmerjahn, F., & Ravetch, J. V. (2005). Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science, 310, 1510–1512.
Vu, T., & Claret, F. X. (2012). Trastuzumab: Updated mechanisms of action and resistance in breast cancer. Frontiers in Oncology, 2, 62.
Bruhns, P. (2012). Properties of mouse and human IgG receptors and their contribution to disease models. Blood, 119, 5640–5649.
Steffens, M. G., Boerman, O. C., Oosterwijk-Wakka, J. C., Oosterhof, G. O., Witjes, J. A., Koenders, E. B., Oyen, W. J., Buijs, W. C., Debruyne, F. M., Corstens, F. H., & Oosterwijk, E. (1997). Targeting of renal cell carcinoma with iodine-131-labeled chimeric monoclonal antibody G250. Journal of Clinical Oncology, 15, 1529–1537.
Gut, M. O., Parkkila, S., Vernerova, Z., Rohde, E., Zavada, J., Hocker, M., Pastorek, J., Karttunen, T., Gibadulinova, A., Zavadova, Z., Knobeloch, K. P., Wiedenmann, B., Svoboda, J., Horak, I., & Pastorekova, S. (2002). Gastric hyperplasia in mice with targeted disruption of the carbonic anhydrase gene Car9. Gastroenterology, 123, 1889–1903.
Leppilampi, M., Karttunen, T. J., Kivela, J., Gut, M. O., Pastorekova, S., Pastorek, J., & Parkkila, S. (2005). Gastric pit cell hyperplasia and glandular atrophy in carbonic anhydrase IX knockout mice: Studies on two strains C57/BL6 and BALB/C. Transgenic Research, 14, 655–663.
Lou, Y., Preobrazhenska, O., auf dem Keller, U., Sutcliffe, M., Barclay, L., McDonald, P. C., Roskelley, C., Overall, C. M., & Dedhar, S. (2008). Epithelial-mesenchymal transition (EMT) is not sufficient for spontaneous murine breast cancer metastasis. Developmental Dynamics, 237, 2755–2768.
Pulaski, B. A., & Ostrand-Rosenberg, S. (2001). Mouse 4T1 breast tumor model. Current Protocols in Immunology. https://doi.org/10.1002/0471142735.im2002s39
Czajka-Francuz, P., Prendes, M. J., Mankan, A., Quintana, A., Pabla, S., Ramkissoon, S., Jensen, T. J., Peiro, S., Severson, E. A., Achyut, B. R., Vidal, L., Poelman, M., & Saini, K. S. (2023). Mechanisms of immune modulation in the tumor microenvironment and implications for targeted therapy. Frontiers in Oncology, 13, 1200646.
Zheng, Y., Li, S., Tang, H., Meng, X., & Zheng, Q. (2023). Molecular mechanisms of immunotherapy resistance in triple-negative breast cancer. Frontiers in Immunology, 14, 1153990.
Chafe, S. C., McDonald, P. C., Saberi, S., Nemirovsky, O., Venkateswaran, G., Burugu, S., Gao, D., Delaidelli, A., Kyle, A. H., Baker, J. H. E., Gillespie, J. A., Bashashati, A., Minchinton, A. I., Zhou, Y., Shah, S. P., & Dedhar, S. (2019). Targeting hypoxia-induced carbonic anhydrase IX enhances immune-checkpoint blockade locally and systemically. Cancer Immunology Research, 7, 1064–1078.
Giatromanolaki, A., Harris, A. L., Banham, A. H., Contrafouris, C. A., & Koukourakis, M. I. (2020). Carbonic anhydrase 9 (CA9) expression in non-small-cell lung cancer: Correlation with regulatory FOXP3+T-cell tumour stroma infiltration. British Journal of Cancer, 122, 1205–1210.
Chafe, S. C., Lou, Y., Sceneay, J., Vallejo, M., Hamilton, M. J., McDonald, P. C., Bennewith, K. L., Moller, A., & Dedhar, S. (2015). Carbonic anhydrase IX promotes myeloid-derived suppressor cell mobilization and establishment of a metastatic niche by stimulating G-CSF production. Cancer Research, 75, 996–1008.
Acknowledgements
The authors declare no competing interests. We are thankful to Dr. Juris Jansons for his technical support with electron microscopy. This study was supported by the European Regional Development Fund (ERDF) grant number 1.1.1.2/VIAA/4/20/728.
Funding
European Regional Development Fund,1.1.1.2/VIAA/4/20/728, Zane Kalniņa.
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to the manuscript as described: conceptualisation: KT and ZK; methodology: ZK, IL, IA and AK; formal analysis and investigation: ZK, IL and VS; funding acquisition: ZK; project administration: ZK; supervision: KT; writing—original draft preparation: ZK and IL; writing—review and editing: all authors; visualisation: ZK.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kalniņa, Z., Liekniņa, I., Skeltona, V. et al. Preclinical Evaluation of virus-like particle Vaccine Against Carbonic Anhydrase IX Efficacy in a Mouse Breast Cancer Model System. Mol Biotechnol 66, 1206–1219 (2024). https://doi.org/10.1007/s12033-023-01021-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-01021-5