Abstract
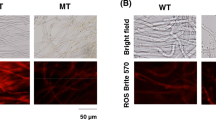
AgHST1 and AgHST3 genes encode sirtuins that are NAD+-dependent protein deacetylases. According to previous reports, their disruption leads to the overproduction of riboflavin in Ashbya gossypii. In this study, we investigated the potential causes of riboflavin overproduction in the AgHST1Δ and AgHST3Δ mutant strains of A. gossypii. The generation of reactive oxygen species was increasd in the mutants compared to in WT. Additionally, membrane potential was lower in the mutants than in WT. The NAD+/NADH ratio in AgHST1Δ mutant strain was lower than that in WT; however, the NAD+/NADH ratio in AgHST3Δ was slightly higher than that in WT. AgHST1Δ mutant strain was more sensitive to high temperatures and hydroxyurea treatment than WT or AgHST3Δ. Expression of the AgGLR1 gene, encoding glutathione reductase, was substantially decreased in AgHST1Δ and AgHST3Δ mutant strains. The addition of N-acetyl-L-cysteine, an antioxidant, suppressed the riboflavin production in the mutants, indicating that it was induced by oxidative stress. Therefore, high oxidative stress resulting from the disruption of sirtuin genes induces riboflavin overproduction in AgHST1Δ and AgHST3Δ mutant strains. This study established that oxidative stress is an important trigger for riboflavin overproduction in sirtuin gene-disrupted mutant strains of A. gossypii and helped to elucidate the mechanism of riboflavin production in A. gossypii.






Similar content being viewed by others
Data Availability
All data and materials analyzed in this study are shown in this published article.
References
You, J., Pan, X., Yang, C., Du, Y., Osire, T., Yang, T., Zhang, X., Xu, M., Xu, G., & Rao, Z. (2021). Microbial production of riboflavin: Biotechnological advances and perspectives. Metabolic Engineering, 68, 46–58.
Zhao, G., Dong, F., Lao, X., & Zheng, H. (2021). Strategies to increase the production of biosynthetic riboflavin. Molecular Biotechnology, 63, 909–918.
Gudipati, V., Koch, K., Lienhart, W. D., & Macheroux, P. (2014). The flavoproteome of the yeast Saccharomyces cerevisiae. Biochimie et Biophysica Acta, 1844, 535–544.
Lienhart, W. D., Gudipati, V., & Macheroux, P. (2013). The human flavoproteome. Archives of Biochemistry and Biophysics, 535, 150–162.
Suwannasom, N., Kao, I., Pruß, A., Georgieva, R., & Bäumler, H. (2020). Riboflavin: The health benefits of a forgotten natural vitamin. International Journal of Molecular Sciences, 21, 950.
Udhayabanu, T., Manole, A., Rajeshwari, M., Varalakshmi, P., Houlden, H., & Ashokkumar, B. (2017). Riboflavin responsive mitochondrial dysfunction in neurodegenerative diseases. Journal of Clinical Medicine, 6, 52.
Ashoori, M., & Saedisomeolia, A. (2014). Riboflavin (vitamin B2) and oxidative stress: A review. British Journal of Nutrition, 111, 1985–1991.
Olfat, N., Ashoori, M., & Saedisomeolia, A. (2022). Riboflavin is an antioxidant: A review update. British Journal of Nutrition, 128, 1887–11895.
Walther, A., & Wendland, J. (2012). Yap1-dependent oxidative stress response provides a link to riboflavin production in Ashbya gossypii. Fungal Genetics and Biology, 49, 697–707.
Silva, R., Aguiar, T. Q., Oliveira, R., & Domingues, L. (2019). Light exposure during growth increases riboflavin production, reactive oxygen species accumulation and DNA damage in Ashbya gossypii riboflavin-overproducing strains. FEMS Yeast Research, 19(1), foy114.
Anam, K., Nasuno, R., & Takagi, H. (2020). A novel mechanism for nitrosative stress tolerance dependent on GTP cyclohydrolase II activity involved in riboflavin synthesis of yeast. Scientific Reports, 10, 6015.
Chen, X., Ji, B., Hao, X., Li, X., Eisele, F., Nyström, T., & Petranovic, D. (2020). FMN reduces amyloid-β toxicity in yeast by regulating redox status and cellular metabolism. Nature Communications, 11, 867.
Gumá-Cintrón, Y., Bandyopadhyay, A., Rosado, W., Shu-Hu, W., & Nadathur, G. S. (2015). Transcriptomic analysis of cobalt stress in the marine yeast Debaryomyces hansenii. FEMS Yeast Research. https://doi.org/10.1093/femsyr/fov099
Boretsky, Y. R., Protchenko, O. V., Prokopiv, T. M., Mukalov, I. O., Fedorovych, D. V., & Sibirny, A. A. (2007). Mutations and environmental factors affecting regulation of riboflavin synthesis and iron assimilation also cause oxidative stress in the yeast Pichia guilliermondii. Journal of Basic Microbiology, 47, 371–377.
Kato, T., Azegami, J., Kano, M., El Enshasy, H. A., & Park, E. Y. (2021). Effects of sirtuins on the riboflavin production in Ashbya gossypii. Applied Microbiology and Biotechnology, 105, 7813–7823.
Houtkooper, R. H., Pirinen, E., & Auwerx, J. (2012). Sirtuins as regulators of metabolism and healthspan. Nature Reviews Molecular Cell Biology, 13, 225–238.
Shahgaldi, S., & Kahmini, F. R. (2021). A comprehensive review of sirtuins: With a major focus on redox homeostasis and metabolism. Life Sciences, 282, 119803.
Wierman, M. B., & Smith, J. S. (2014). Yeast sirtuins and the regulation of aging. FEMS Yeast Research, 14, 73–88.
Singh, C. K., Chhabra, G., Ndiaye, M. A., Garcia-Peterson, L. M., Mack, N. J., & Ahmad, N. (2018). The role of sirtuins in antioxidant and redox signaling. Antioxidants and Redox Signaling, 28, 643–661.
Srivastava, S. (2016). Emerging therapeutic roles for NAD+ metabolism in mitochondrial and age-related disorders. Clinical and Translational Medicine, 5, 25.
Shimizu, K., & Matsuoka, Y. (2019). Redox rebalance against genetic perturbations and modulation of central carbon metabolism by the oxidative stress regulation. Biotechnology Advances, 37, 107441.
Stein, L. R., & Imai, S. (2012). The dynamic regulation of NAD metabolism in mitochondria. Trends in Endocrinology & Metabolism, 23, 420–428.
Kim, J. K., Park, J., Ryu, T. H., & Nili, M. (2013). Effect of N-acetyl-L-cysteine on Saccharomyces cerevisiae irradiated with gamma-rays. Chemosphere, 92, 512–516.
Huang, M. E., Facca, C., Fatmi, Z., Baïlle, D., Bénakli, S., & Vernis, L. (2016). DNA replication inhibitor hydroxyurea alters Fe-S centers by producing reactive oxygen species in vivo. Scientific Reports, 6, 29361.
Singh, A., & Xu, Y. J. (2016). The cell killing mechanisms of hydroxyurea. Genes (Basel), 7, 99.
Singh, A., & Xu, Y. J. (2017). Heme deficiency sensitizes yeast cells to oxidative stress induced by hydroxyurea. Journal of Biological Chemistry, 292, 9088–9103.
Oscarsson, T., Walther, A., Lengeler, K. B., & Wendland, J. (2017). An Arf-GAP promotes endocytosis and hyphal growth of Ashbya gossypii. FEMS Microbiology Letters. https://doi.org/10.1093/femsle/fnx240
Fedoseeva, I. V., Pyatrikas, D. V., Stepanov, A. V., Fedyaeva, A. V., Varakina, N. N., Rusaleva, T. M., Borovskii, G. B., & Rikhvanov, E. G. (2017). The role of flavin-containing enzymes in mitochondrial membrane hyperpolarization and ROS production in respiring Saccharomyces cerevisiae cells under heat-shock conditions. Scientific Reports, 7, 2586.
Chen, J., Zhai, W., Li, Y., Guo, Y., Zhu, Y., & Lei, & G., Li, J. (2022). Enhancing the biomass and riboflavin production of Ashbya gossypii by using low-intensity ultrasound stimulation. Biochemical Engineering Journal, 181, 108394.
Kavitha, S., & Chandra, T. S. (2014). Oxidative stress protection and glutathione metabolism in response to hydrogen peroxide and menadione in riboflavinogenic fungus Ashbya gossypii. Applied Biochemistry and Biotechnology, 174, 2307–2325.
Cheung, I. M., McGhee, C. N., & Sherwin, T. (2014). Beneficial effect of the antioxidant riboflavin on gene expression of extracellular matirix elements, antioxidants and oxidases in keratoconic stromal cells. Clinical and Experimental Optometry, 97, 349–355.
Sanches, S. C., Ramalho, L. N., Mendes-Braz, M., Terra, V. A., Cecchini, R., Augusto, M. J., & Ramalho, F. S. (2014). Riboflavin (vitamin B-2) reduces hepatocellular injury following liver ischaemia and reperfusion in mice. Food and Chemical Toxicology, 67, 65–71.
Farah, N., Chin, V. K., Chong, P. P., Lim, W. F., Lim, C. W., Basir, R., Chang, S. K., & Lee, T. Y. (2022). Riboflavin as a promising antimicrobial agent? A multi-perspective review. Current Research in Microbial Sciences, 3, 100111.
Balaban, B. G., Yılmaz, Ü., Alkım, C., Topaloğlu, A., Kısakesen, H. İ, Holyavkin, C., & Çakar, Z. P. (2019). Evolutionary engineering of an iron-resistant Saccharomyces cerevisiae mutant and its physiological and molecular characterization. Microorganisms, 8, 43.
Kocaefe-Özşen, N., Yilmaz, B., Alkım, C., Arslan, M., Topaloğlu, A., Kısakesen, H. L. B., Gülsev, E., & Çakar, Z. P. (2022). Physiological and molecular characterization of an oxidative stress-resistant Saccharomyces cerevisiae strain obtained by evolutionary engineering. Frontiers in Microbiology, 13, 822864.
Merksamer, P. I., Liu, Y., He, W., Hirschey, M. D., Chen, D., & Verdin, E. (2013). The sirtuins, oxidative stress and aging: An emerging link. Aging (Albany NY), 5, 144–150.
Alam, F., Syed, H., Amjad, S., Baig, M., Khan, T. A., & Rehman, R. (2021). Interplay between oxidative stress, SIRT1, reproductive and metabolic functions. Current Research in Physiology, 4, 119–124.
Wan, X., & Garg, N. J. (2021). Sirtuin control of mitochondrial dysfunction, oxidative stress, and inflammation in chagas disease models. Frontiers in Cellular and Infection Microbiology, 11, 693051.
Bause, A. S., & Haigis, M. C. (2013). SIRT3 regulation of mitochondrial oxidative stress. Experimental Gerontology, 48, 634–639.
Collins, J. A., Kapustina, M., Bolduc, J. A., Pike, J. F. W., Diekman, B. O., Mix, K., Chubinskaya, S., Eroglu, E., Michel, T., Poole, L. B., Furdui, C. M., & Loeser, R. F. (2021). Sirtuin 6 (SIRT6) regulates redox homeostasis and signaling events in human articular chondrocytes. Free Radical Biology and Medicine, 166, 90–103.
Liao, C. Y., & Kennedy, B. K. (2016). SIRT6, oxidative stress, and aging. Cell Research, 26, 143–144.
Manthey, K. C., Rodriguez-Melendez, R., Hoi, J. T., & Zempleni, J. (2006). Riboflavin deficiency causes protein and DNA damage in HepG2 cells, triggering arrest in G1 phase of the cell cycle. The Journal of Nutritional Biochemistry, 17, 250–256.
Song, J. Y., Cha, J., Lee, J., & Roe, J. H. (2006). Glutathione reductase and a mitochondrial thioredoxin play overlapping roles in maintaining iron-sulfur enzymes in fission yeast. Eukaryotic Cells, 5, 1857–1865.
Chen, J., Shen, J., Solem, C., & Jensen, P. R. (2013). Oxidative stress at high temperatures in Lactococcus lactis due to an insufficient supply of riboflavin. Applied and Environmental Microbiology, 79, 6140–6147.
Fabrizio, P., Gattazzo, C., Battistella, L., Wei, M., Cheng, C., McGrew, K., & Longo, V. D. (2005). Sir2 blocks extreme life-span extension. Cell, 123, 655–667.
Vall-Llaura, N., Mir, N., Garrido, L., Vived, C., & Cabiscol, E. (2019). Redox control of yeast Sir2 activity is involved in acetic acid resistance and longevity. Redox Biology, 24, 101229.
Knieß, R. A., & Mayer, M. P. (2016). The oxidation state of the cytoplasmic glutathione redox system does not correlate with replicative lifespan in yeast. NPJ Aging and Mechanisms of Disease, 2, 16028.
Acknowledgements
We would like to thank Kohei Kurabayashi (Department of Applied Life Science, Faculty of Agriculture, Shizuoka University) to help our response to reviewers with additional experiments.
Funding
This study was supported by JSPS KAKENHI (Grant-in-Aid for Scientific Research (C); Grant Number JP21K05390).
Author information
Authors and Affiliations
Contributions
Study conception and design were performed by TK, HAEE, and EYP. Material preparation, data collection, and analysis were performed by JA, MK, HAEE, and EYP. The first draft of the manuscript was written by TK, HAEE and EYP. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors have read and approved the manuscript for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kato, T., Azegami, J., Kano, M. et al. Induction of Oxidative Stress in Sirtuin Gene-Disrupted Ashbya gossypii Mutants Overproducing Riboflavin. Mol Biotechnol 66, 1144–1153 (2024). https://doi.org/10.1007/s12033-023-01012-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-01012-6