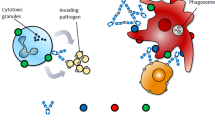
Abstract
Since the advent of hybridoma technology in the year 1975, it took a decade to witness the first approved monoclonal antibody Orthoclone OKT39 (muromonab-CD3) in the year 1986. Since then, continuous strides have been made to engineer antibodies for specific desired effects. The engineering efforts were not confined to only the variable domains of the antibody but also included the fragment crystallizable (Fc) region that influences the immune response and serum half-life. Engineering of the Fc fragment would have a profound effect on the therapeutic dose, antibody-dependent cell-mediated cytotoxicity as well as antibody-dependent cellular phagocytosis. The integration of computational techniques into antibody engineering designs has allowed for the generation of testable hypotheses and guided the rational antibody design framework prior to further experimental evaluations. In this article, we discuss the recent works in the Fc-fused molecule design that involves computational techniques. We also summarize the usefulness of in silico techniques to aid Fc-fused molecule design and analysis for the therapeutics application.

Similar content being viewed by others
References
Huber, R., Deisenhofer, J., Colman, P. M., Matsushima, M., & Palm, W. (1976). Crystallographic structure studies of an IgG molecule and an Fc fragment. Nature, 264, 415–420.
Nisonoff, A., Wissler, F. C., Lipman, L. N., & Woernley, D. L. (1960). Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Archives of Biochemistry and Biophysics, 89, 230–244.
Padlan, E. A., Davies, D. R., Rudikoff, S., & Potter, M. (1976). Structural basis for the specificity of phosphorylcholine-binding immunoglobulins. Immunochemistry, 13, 945–949.
Saxena, A., & Wu, D. (2016). Advances in therapeutic Fc engineering—Modulation of IgG-associated effector functions and serum half-life. Frontiers in Immunology, 7, 580.
Park, H. I., Yoon, H. W., & Jung, S. T. (2016). The highly evolvable antibody Fc domain. Trends in Biotechnology, 34, 895–908.
Dong, J., Huang, B., Jia, Z., Wang, B., Gallolu Kankanamalage, S., Titong, A., & Liu, Y. (2020). Development of multi-specific humanized llama antibodies blocking SARS-CoV-2/ACE2 interaction with high affinity and avidity. Emerging Microbes & Infections, 9, 1034–1036.
Dong, J., Huang, B., Wang, B., Titong, A., Gallolu Kankanamalage, S., Jia, Z., Wright, M., Parthasarathy, P., & Liu, Y. (2020). Development of humanized tri-specific nanobodies with potent neutralization for SARS-CoV-2. Science and Reports, 10, 17806.
Doosti, M., Nassiri, M., Nasiri, K., Tahmoorespur, M., & Zibaee, S. (2019). Immunogenic evaluation of FMD virus immuno-dominant epitopes coupled with IL-2/FcIgG in BALB/c mice. Microbial Pathogenesis, 132, 30–37.
Su, C.T.-T., Lua, W.-H., Ling, W.-L., & Gan, S.K.-E. (2018). Allosteric effects between the antibody constant and variable regions: A study of IgA Fc mutations on antigen binding. Antibodies, 7, 20.
Lazar, G. A., Dang, W., Karki, S., Vafa, O., Peng, J. S., Hyun, L., Chan, C., Chung, H. S., Eivazi, A., Yoder, S. C., Vielmetter, J., Carmichael, D. F., Hayes, R. J., & Dahiyat, B. I. (2006). Engineered antibody Fc variants with enhanced effector function. Proceedings of the National Academy of Sciences of the United States of America, 103, 4005–4010.
Jebamani, P., Sriramulu, D. K., & Lee, S.-G. (2023). Residue interaction network and molecular dynamics simulation study on the binding of S239D/I332E Fc variant with enhanced affinity to FcγRIIIa receptor. Journal of Molecular Graphics and Modelling, 118, 1–9.
Schmied, B. J., Lutz, M. S., Riegg, F., Zekri, L., Heitmann, J. S., Bühring, H.-J., Jung, G., & Salih, H. R. (2019). Induction of NK cell reactivity against B-cell acute lymphoblastic leukemia by an Fc-optimized FLT3 antibody. Cancers, 11, 1–15.
Schmied, B. J., Riegg, F., Zekri, L., Grosse-Hovest, L., Bühring, H.-J., Jung, G., & Salih, H. R. (2019). An Fc-optimized CD133 antibody for induction of natural killer cell reactivity against colorectal cancer. Cancers, 11, 1–13.
Edwards, J. M., Heydarchi, B., Khoury, G., Salazar-Quiroz, N. A., Gonelli, C. A., Wines, B., Hogarth, P. M., Kristensen, A. B., Parsons, M. S., & Purcell, D. F. J. (2021). Enhancement of antibody-dependent cellular cytotoxicity and phagocytosis in anti-HIV-1 human-bovine chimeric broadly neutralizing antibodies. Journal of Virology, 95, e0021921.
Babaki, M. K. Z., Taghiabadi, M., Soleimanpour, S., Moghadam, M. S., Mosavat, A., Amini, A. A., Mohammadi, M. A., & Rezaee, S. A. (2018). Mycobacterium tuberculosis Ag85b:Hfcγ1 recombinant fusion protein as a selective receptor-dependent delivery system for antigen presentation. Microbial Pathogenesis, 129, 68–73.
Deisenhofer, J. (1981). Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry, 20, 2361–2370.
Colovos, C., & Yeates, T. O. (1993). Verification of protein structures: Patterns of nonbonded atomic interactions. Protein Science, 2, 1511–1519.
Laskowski, R. A., MacArthur, M. W., Moss, D. S., & Thornton, J. M. (1993). PROCHECK: A program to check the stereochemical quality of protein structures. Journal of Applied Crystallography, 26, 283–291.
Divine, R., Dang, H. V., Ueda, G., Fallas, J. A., Vulovic, I., Sheffler, W., Saini, S., Zhao, Y. T., & Raj, I. X. (2021). Designed protein assemble antibodies into modular nanocages. Science, 372, 1–17.
Malito, E., Surdo, P. L., Veggi, D., Santini, L., Stefek, H., Brunelli, B., Luzzi, E., Bottomley, M. J., Beernink, P. T., & Scarselli, M. (2016). Neisseria meningitidis factor H binding protein bound to monoclonal antibody JAR5: Implications for antibody synergy. The Biochemical Journal, 473, 4699–4713.
Kaplan, W., & Littlejohn, T. G. (2001). Swiss-PDB viewer (deep view). Briefings in Bioinformatics, 2, 195–197.
Gallagher, D. T., Galvin, C. V., & Karageorgos, I. (2018). Structure of the Fc fragment of the NIST reference antibody RM8671. Acta Crystallographica Section F: Structural Biology Communications, 74, 524–529.
DeLano, W. L. (2003). The PyMOL molecular graphics system. DeLano Scientic LLC.
Krissinel, E., & Henrick, K. (2007). Inference of macromolecular assemblies from crystalline state. Journal of Molecular Biology, 372, 774–797.
Zhu, K., Day, T., Warshaviak, D., Murrett, C., Friesner, R., & Pearlman, D. (2014). Antibody structure determination using a combination of homology modeling, energy-based refinement, and loop prediction. Proteins, 82, 1646–1655.
Belviso, B. D., Mangiatordi, G. F., Alberga, D., Mangini, V., Carrozzini, B., & Caliandro, R. (2022). Structural characterization of the full-length anti-CD20 antibody rituximab. Frontiers in Molecular Biosciences, 9, 1–18.
Jumper, J., Evans, R., Pritzel, A., Green, T., Figurnov, M., & Ronneberger, O. (2021). Highly accurate protein structure prediction with alphafold. Nature, 596, 583–589.
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W., & Klein, M. L. (1983). Comparison of simple potential functions for simulating liquid water. The Journal of Chemical Physics, 79, 926–935.
Phillips, J. C., Hardy, D. J., Maia, J. D. C., Stone, J. E., Ribeiro, J. V., Bernardi, R. C., Buch, R., Fiorin, G., Henin, J., Jiang, W., McGreevy, R., Melo, M. C. R., Radak, B. K., Skeel, R. D., Singharoy, A., Wang, Y., Roux, B., Aksimentiev, A., Luthey-Schulten, Z., … Tajkhorshid, E. (2020). Scalable molecular dynamics on CPU and GPU architectures with NAMD. The Journal of Chemical Physics, 153, 044130.
Best, R. B., Zhu, X., Shim, J., Lopes, P. E. M., Mittal, J., & Feig, M. (2012). Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone ϕ, ψ and side chain χ1 and χ2 dihedral angles. Journal of Chemical Theory and Computation, 8, 3257–3273.
Ingrama, J. R., Blombergb, O. S., Rashidianb, M., Alia, L., Garforthc, S., Fedorovc, E., Fedorovc, A. A., Bonannoc, J. B., Le Gallb, C., Crowleya, S., Espinosaa, C., Kelihere, E. J., Weissledere, R., Almo, S. C., Dougan, S. K., Ploegh, H. L., & Dougan, M. (2018). Anti-CTLA-4 therapy requires an Fc domain for efficacy. Proceedings of the National Academy of Sciences of the United States of America, 115, 3912–3917.
Linsley, P. S., Greene, J. L., Brady, W., Bajorath, J., Ledbetter, J. A., & Peach, R. (1994). Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity, 1, 793–801.
Stamper, C. C., Zhang, Y., Tobin, J. F., Erbe, D. V., Ikemizu, S., Davis, S. J., Stahl, M. L., Seehra, J., Somers, W. S., & Mosyak, L. (2001). Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature, 410, 608–611.
Egen, J. G., Kuhns, M. S., & Allison, J. P. (2002). CTLA-4: New insights into its biological function and use in tumor immunotherapy. Nature Immunology, 3, 611–618.
Emsley, P., Lohkamp, B., Scott, W. G., & Cowtan, K. (2010). Features and development of Coot. Acta Crystallographica Section D: Biological Crystallography, 66, 486–501.
Adams, P. D., Afonine, P. V., Bunkoczi, G., Chen, V. B., Davis, I. W., Echols, N., & Headd, J. J. (2010). PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallographica Section D: Biological Crystallography, 66, 213–221.
Zhang, L., She, H., Gong, Y., Pang, X., Yi, M., Guo, L., Li, J., Arroyo, S., Lu, X., Ovchinnikov, S., Cheng, G., Liu, X., Jiang, X., Feng, S., & Deng, H. (2019). Development of a dual-functional conjugate of antigenic peptide and Fc-III mimetics (DCAF) for targeted antibody blocking. Chemical Science, 10, 3271–3280.
Cavaco, M., Castanho, M., & Neves, V. (2017). Peptibodies: An elegant solution for a long-standing problem. Biopolymers, 110, e23095.
Shimamoto, G., Gegg, C., Boone, T., & Queva, C. (2012). Peptibodies: A flexible alternative format to antibodies. MAbs, 4, 586–591.
Zhao, Y., Hao, X., Feng, J., Shen, B., Wei, J., & Sun, J. (2015). The comparison of BLyS-binding peptides from phage display library and computer-aided design on BLyS-TACI interaction. International Immunopharmacology, 24, 219–223.
Zhu, W., Sun, X., Zhu, L., Gan, Y., Baiwu, R., Wei, J., Li, Z., Li, R., & Sun, J. (2016). A novel BLyS peptibody down-regulates B cell and T helper cell subsets in vivo and ameliorates collagen-induced arthritis. Inflammation, 39, 839–848.
Lim, Y. Y., Lim, T. S., & Choong, Y. S. (2020). Human IgG1 Fc pH-dependent optimization from a constant pH molecular dynamics simulation analysis. RSC Advances, 10, 13066–13075.
Dall’Acqua, W. F., Kiener, P. A., & Wu, H. (2006). Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). Journal of Biological Chemistry, 281, 23514–23524.
Mackness, B. C., Jaworski, J. A., Boudanova, E., Park, A., Valente, D., Mauriac, C., Pasquier, O., Schmidt, T., Kabiri, M., Kandira, A., Radošević, K., & Qiu, H. (2019). Antibody Fc engineering for enhanced neonatal Fc receptor binding and prolonged circulation half-life. MAbs, 11, 1276–1288.
Zhang, Y., He, B., Liu, K., Ning, L., Luo, D., Xu, K., Zhu, W., Wu, Z., Huang, J., & Xu, X. (2017). A novel peptide specifically binding to VEGF receptor suppresses angiogenesis in vitro and in vivo. Signal Transduction and Targeted Therapy, 2, 17010.
Ning, L., Li, Z., Bai, Z., Hou, S., He, B., Huang, J., & Zhou, P. (2018). Computational design of antiangiogenic peptibody by fusing human IgG1 Fc fragment and HRH peptide: Structural modeling, energetic analysis, and dynamics simulation of its binding potency to VEGF receptor. International Journal of Biological Sciences, 14, 930–937.
Trabuco, L. G., Lise, S., Petsalaki, E., & Russell, R. B. (2012). PepSite: Prediction of peptide-binding sites from protein surfaces. Nucleic Acids Research, 40, W423–W427.
Maupetit, J., Derreumaux, P., & Tuffery, P. (2009). PEP-FOLD: An online resource for de novo peptide structure prediction. Nucleic Acids Research, 37, W498–W503.
Chen, R., Li, L., & Weng, Z. (2003). ZDOCK: An initial-stage protein-docking algorithm. Proteins, 52, 80–87.
Duan, Y., Wu, C., Chowdhury, S., Lee, M. C., Xiong, G., Zhang, W., Yang, R., Cieplak, P., Luo, R., Lee, T., Caldwell, J., Wang, J., & Kollman, P. (2003). A point-charge force field for molecular mechanics simulations of proteins. Journal of Computational Chemistry, 24, 1999–2012.
Cottignies-Calamarte, A., Tudor, D., & Morgane, B. (2023). Antibody Fc-chimerism and effector functions: When IgG takes advantage of IgA. Frontiers in Immunology, 14, 1–16.
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410.
Sievers, F., Wilm, A., Dineen, D., Gibson, T. J., Karplus, K., Li, W., Lopez, R., McWilliam, H., Remmert, M., Soding, J., Thompson, J. D., & Higgins, D. G. (2011). Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular Systems Biology, 7, 539.
Lassmann, T. (2019). Kalign 3: Multiple sequence alignment of large data sets. Bioinformatics, 36, 1928–1929.
Jackman, S. D., Vandervalk, B. P., Mohamadi, H., Chu, J., Yeo, S., Hammond, S. A., Jahesh, G., Khan, H., Coombe, L., Warren, R. L., & Birol, I. (2017). ABySS 2.0: Resource-efficient assembly of large genomes using a Bloom filter. Genome Research, 27, 768–777.
Steinegger, M., Mirdita, M., & Soding, J. (2019). Protein-level assembly increases protein sequence recovery from metagenomic samples manyfold. Nature Methods, 16, 603–606.
Prjibelski, A., Antipov, D., Meleshko, D., Lapidus, A., & Korobeynikov, A. (2020). Using SPAdes de novo assembler. Current Protocols in Bioinformatics, 70, e102.
Pettersen, E. F., Goddard, T. D., Huang, C. C., Meng, E. C., Couch, G. S., Croll, T. I., Morris, J. H., & Ferrin, T. E. (2021). UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Science, 30, 70–82.
Lee, B. D., Timony, M. A., & Ruiz, P. (2019). DNAvisualization.org: A serverless web tool for DNA sequence visualization. Nucleic Acids Research, 47, W20–W25.
Lemoine, F., Correia, D., Lefort, V., Doppelt-Azeroual, O., Mareuil, F., Cohen-Boulakia, S., & Gascuel, O. (2019). NGPhylogeny.fr: New generation phylogenetic services for non-specialists. Nucleic Acids Research, 47, W260–W265.
Jespersen, M. C., Peters, B., Nielsen, M., & Marcatili, P. (2017). BepiPred-2.0: Improving sequence-based B-cell epitope prediction using conformational epitopes. Nucleic Acids Research, 45, W24–W29.
Ponomarenko, J., Bui, H. H., Li, W., Fusseder, N., Bourne, P. E., Sette, A., & Peters, B. (2008). ElliPro: A new structure-based tool for the prediction of antibody epitopes. BMC Bioinformatics, 9, 514.
Vita, R., Overton, J. A., Greenbaum, J. A., Ponomarenko, J., Clark, J. D., Cantrell, J. R., Wheeler, D. K., Gabbard, J. L., Hix, D., Sette, A., & Peters, B. (2015). The immune epitope database (IEDB) 3.0. Nucleic Acids Research, 43, D405–D412.
Claude, J. B., Suhre, K., Notredame, C., Claverie, J. M., & Abergel, C. (2004). CaspR: A web server for automated molecular replacement using homology modelling. Nucleic Acids Research, 32, W606–W609.
Wang, Y., Virtanen, J., Xue, Z., & Zhang, Y. (2017). I-TASSER-MR: Automated molecular replacement for distant-homology proteins using iterative fragment assembly and progressive sequence truncation. Nucleic Acids Research, 45, W429–W434.
Virtanen, J. J., & Zhang, Y. (2018). MR-REX: Molecular replacement by cooperative conformational search and occupancy optimization on low-accuracy protein models. Acta Crystallographica Section D: Structural Biology, 74, 606–620.
Croll, T. I. (2018). ISOLDE: A physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallographica Section D: Structural Biology, 74, 519–530.
Turk, D. (2013). MAIN software for density averaging, model building, structure refinement and validation. Acta Crystallographica Section D: Biological Crystallography, 69, 1342–1357.
Terwilliger, T. C., Dimaio, F., Read, R. J., Baker, D., Bunkoczi, G., Adams, P. D., Grosse-Kunstleve, R. W., Afonine, P. V., & Echols, N. (2012). phenix.mr_rosetta: Molecular replacement and model rebuilding with Phenix and Rosetta. Journal of Structural and Functional Genomics, 13, 81–90.
Murshudov, G. N., Skubak, P., Lebedev, A. A., Pannu, N. S., Steiner, R. A., Nicholls, R. A., Winn, M. D., Long, F., & Vagin, A. A. (2011). REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallographica Section D: Biological Crystallography, 67, 355–367.
Sheldrick, G. M. (2008). A short history of SHELX. Acta Crystallographica, 64, 112–122.
Chen, V. B., Arendall, W. B., 3rd., Headd, J. J., Keedy, D. A., Immormino, R. M., Kapral, G. J., Murray, L. W., Richardson, J. S., & Richardson, D. C. (2010). MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallographica Section D: Biological Crystallography, 66, 12–21.
Berman, H., Henrick, K., & Nakamura, H. (2003). Announcing the worldwide Protein Data Bank. Natural Structural Biology, 10, 980.
Jin, S., Contessoto, V. G., Chen, M., Schafer, N. P., Lu, W., Chen, X., Bueno, C., Hajitaheri, A., Sirovetz, B. J., Davtyan, A., Papoian, G. A., Tsai, M. Y., & Wolynes, P. G. (2020). AWSEM-Suite: A protein structure prediction server based on template-guided, coevolutionary-enhanced optimized folding landscapes. Nucleic Acids Research, 48, W25–W30.
Sali, A., & Blundell, T. L. (1993). Comparative protein modelling by satisfaction of spatial restraints. Journal of Molecular Biology, 234, 779–815.
Waterhouse, A., Bertoni, M., Bienert, S., Studer, G., Tauriello, G., Gumienny, R., Heer, F. T., de Beer, T. A. P., Rempfer, C., Bordoli, L., Lepore, R., & Schwede, T. (2018). SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Research, 46, W296–W303.
Greener, J. G., Kandathil, S. M., & Jones, D. T. (2019). Deep learning extends de novo protein modelling coverage of genomes using iteratively predicted structural constraints. Nature Communications, 10, 3977.
Xu, D., & Zhang, Y. (2012). Ab initio protein structure assembly using continuous structure fragments and optimized knowledge-based force field. Proteins, 80, 1715–1735.
Kim, D. E., Chivian, D., & Baker, D. (2004). Protein structure prediction and analysis using the Robetta server. Nucleic Acids Research, 32, W526–W531.
Morris, G. M., Huey, R., Lindstrom, W., Sanner, M. F., Belew, R. K., Goodsell, D. S., & Olson, A. J. (2009). AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. Journal of Computational Chemistry, 30, 2785–2791.
Kurcinski, M., Jamroz, M., Blaszczyk, M., Kolinski, A., & Kmiecik, S. (2015). CABS-dock web server for the flexible docking of peptides to proteins without prior knowledge of the binding site. Nucleic Acids Research, 43, W419–W424.
Desta, I. T., Porter, K. A., Xia, B., Kozakov, D., & Vajda, S. (2020). Performance and its limits in rigid body protein–protein docking. Structure, 28, 1071–1081.
Laskowski, R. A., & Swindells, M. B. (2011). LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. Journal of Chemical Information and Modeling, 51, 2778–2786.
Adasme, M. F., Linnemann, K. L., Bolz, S. N., Kaiser, F., Salentin, S., Haupt, V. J., & Schroeder, M. (2021). PLIP 2021: Expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Research, 49(W1), W530–W534.
Case, D. A., Aktulga, H. M., Belfon, K., Ben-Shalom, I. Y., Brozell, S. R., Cerutti, D. S., Cheatham, T. E. I., Cruzeiro, V. M. D., Darden, T. A., Duke, R. E., Giambasu, G., Gilson, M. K., Gohlke, H., Goetz, A. W., Harris, R., Izadi, S., Izmailov, S. A., Jin, C., Kasavajhala, K., … Kollman, P. A. (2021). Amber20. University of California.
Berendsen, H. J. C., van der Spoel, D., & van Drunen, R. (1995). GROMACS: A message-passing parallel molecular dynamics implementation. Computer Physics Communications, 91, 43–56.
Zhang, Y. (2008). I-TASSER server for protein 3D structure prediction. BMC Bioinformatics, 9, 40.
Brown, D. K., Penkler, D. L., Sheik Amamuddy, O., Ross, C., Atilgan, A. R., Atilgan, C., & Tastan Bishop, O. (2017). MD-TASK: A software suite for analyzing molecular dynamics trajectories. Bioinformatics, 33, 2768–2771.
Laaksonen, L. (1992). A graphics program for the analysis and display of molecular dynamics trajectories. Journal of Molecular Graphics, 10, 33–43.
Carrillo-Tripp, M., Alvarez-Rivera, L., Lara-Ramírez, O. I., Becerra-Toledo, F. J., Vega-Ramírez, A., Quijas-Valades, E., González-Zavala, E., González-Vázquez, J. C., García-Vieyra, J., Santoyo-Rivera, N. B., Chapa-Vergara, S. V., & Meneses-Viveros, A. (2018). HTMoL: Full-stack solution for remote access, visualization, and analysis of molecular dynamics trajectory data. Journal of Computer-Aided Molecular Design, 32, 869–876.
Humphrey, W., Dalke, A., & Schulten, K. (1996). VMD: Visual molecular dynamics. Journal of Molecular Graphics, 14, 33–38.
Hunter, S. A., Mclntosh, B. J., Shi, Y., Sperberg, R. A. P., Funatogawa, C., Labanieh, L., Soon, E., Wastyk, H. C., Mehta, N., Carter, C., Hunter, T., & Cochran, J. R. (2021). An engineered ligand trap inhibits leukemi inhibitory factor as pancreatic cancer treatment strategy. Communications Biology, 4, 1–13.
Funding
This work is supported by FRGS (203/CIPPM/6711680; FRGS/1/2018/STG05/USM/02/1) from the Malaysia Ministry of Education.
Author information
Authors and Affiliations
Contributions
CLN drafted the manuscript. TSL and YSC designed and edit the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ng, C.L., Lim, T.S. & Choong, Y.S. Application of Computational Techniques in Antibody Fc-Fused Molecule Design for Therapeutics. Mol Biotechnol 66, 568–581 (2024). https://doi.org/10.1007/s12033-023-00885-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-00885-x