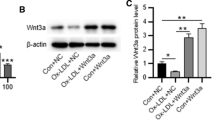
Abstract
Kruppel-like factor 15 (KLF15) is involved in many cardiovascular diseases and is abnormally expressed in atherosclerosis (AS), but the regulatory mechanism of KLF15 in AS has not been reported so far. RT-qPCR was used to detect the expression of KLF15 and ATG14 in AS patients. Subsequently, human aortic endothelial cells (HAECs) were induced by oxidized low densitylipoprotein (ox-LDL), and the expression of KLF15 in model cells was detected. KLF15 was overexpressed in cells by lipofection transfection, and then CCK8, flow cytometry, Western blot, ELISA, and related assay kits were used to detect cell viability, apoptosis, inflammatory response as well as oxidative stress, respectively. The targeted regulatory relationship between KLF15 and autophagy-related 14 (ATG14) was detected by ChIP and luciferase reporter assays. Following ATG14 silencing in KLF15-overexpressing cells, immunofluorescence and Western blot were used to detect the autophagy. Finally, after the addition of 3-Methyladenine (3-MA), an autophagy inhibitor, the aforementioned experiments were conducted again to further explore the mechanism. The expression of KLF15 and ATG14 were decreased in AS patients and ox-LDL-induced HAECs. Overexpression of KLF15 protected ox-LDL-induced HAECs from damage, which might be achieved through transcriptional regulation of ATG14. In addition, KLF15 could promote autophagy through transcriptional activation of ATG14. KLF15 transcriptionally activated ATG14 to promote autophagy and attenuate damage of ox-LDL-induced HAECs.






Similar content being viewed by others
Data Availability
The datasets used and/or analyzed generated during the current study are available from the corresponding author on reasonable request.
References
Libby, P. (2021). The changing landscape of atherosclerosis. Nature, 592, 524–533.
Soehnlein, O., & Libby, P. (2021). Targeting inflammation in atherosclerosis—From experimental insights to the clinic. Nature Reviews. Drug Discovery, 20, 589–610.
Rocha, V. Z., & Libby, P. (2009). Obesity, inflammation, and atherosclerosis. Nature Reviews Cardiology, 6, 399–409.
Gimbrone, M. A., Jr., & Garcia-Cardena, G. (2016). Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circulation Research, 118, 620–636.
Levine, B., & Kroemer, G. (2019). Biological functions of autophagy genes: A disease perspective. Cell, 176, 11–42.
Yuping, Y., Hua, C., & Qing, Z. (2018). Advances in the relationship between Kruppel-like factor 15 and cardiovascular disease research. Cardiovascular Endocrinology & Metabolism, 7, 37–41.
Lu, Y., Zhang, L., Liao, X., Sangwung, P., Prosdocimo, D. A., Zhou, G., Votruba, A. R., Brian, L., Han, Y. J., Gao, H., & Wang, Y. (2013). Kruppel-like factor 15 is critical for vascular inflammation. The Journal of Clinical Investigation, 123, 4232–4241.
Liu, B., Xu, L., Yu, X., Li, W., Sun, X., Xiao, S., Guo, M., & Wang, H. (2018). Protective effect of KLF15 on vascular endothelial dysfunction induced by TNFalpha. Molecular Medicine Reports, 18, 1987.
Nègre-Salvayre, A., Augé, N., Camaré, C., Bacchetti, T., Ferretti, G., & Salvayre, R. (2017). Dual signaling evoked by oxidized LDLs in vascular cells. Free Radical Biology & Medicine, 106, 118–133.
Kattoor, A. J., Kanuri, S. H., & Mehta, J. L. (2019). Role of Ox-LDL and LOX-1 in atherogenesis. Current Medicinal Chemistry, 26, 1693–1700.
Wei, X., Lin, H., Zhang, B., Li, M., Chen, Y., Huang, Y., Zhang, J., Yang, Y., Guo, Z., Li, W., & Ye, L. (2021). Phoenixin-20 prevents ox-LDL-induced attachment of monocytes to human aortic endothelial cells (HAECs): A protective implication in atherosclerosis. ACS Chemical Neuroscience, 12, 990–997.
Gao, L., Guo, Y., Liu, X., Shang, D., & Du, Y. (2017). KLF15 protects against isoproterenol-induced cardiac hypertrophy via regulation of cell death and inhibition of Akt/mTOR signaling. Biochemical and Biophysical Research Communications, 487, 22–7.
Wang, Y., Zhang, C. X., Ge, S. L., & Gong, W. H. (2020). CTBP1AS2 inhibits proliferation and induces autophagy in oxLDLstimulated vascular smooth muscle cells by regulating miR1955p/ATG14. International Journal of Molecular Medicine, 46, 839–848.
Gui, Y., Zheng, H., & Cao, R. Y. (2022). Foam cells in atherosclerosis: novel insights into its origins, consequences, and molecular mechanisms. Frontiers in Cardiovascular Medicine, 9, 845942.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif), 25, 402–8.
Gao, Y., Li, G., Fan, S., Wei, H., Li, M., & Li, X. (2021). Circ_0093887 upregulates CCND2 and SUCNR1 to inhibit the ox-LDL-induced endothelial dysfunction in atherosclerosis by functioning as a miR-876-3p sponge. Clinical and Experimental Pharmacology and Physiology, 48, 1137–1149.
Xu, X. S., Shao, N., Duan, X. T., Zhang, X., & Zhang, Y. (2018). Tacrolimus alleviates Ox-LDL damage through inducing vascular endothelial autophagy. European Review for Medical and Pharmacological Sciences, 22, 3199–3206.
Yu, X. H., Fu, Y. C., Zhang, D. W., Yin, K., & Tang, C. K. (2013). Foam cells in atherosclerosis. Clinica Chimica Acta: International Journal of Clinical Chemistry, 424, 245–52.
Sluiter, T. J., van Buul, J. D., Huveneers, S., Quax, P. H., & de Vries, M. R. (2021). Endothelial barrier function and leukocyte transmigration in atherosclerosis. Biomedicines, 9, 328.
Wei, Z., Ran, H., & Yang, C. (2020). CircRSF1 contributes to endothelial cell growth, migration and tube formation under ox-LDL stress through regulating miR-758/CCND2 axis. Life Sciences, 259, 118241.
Marchio, P., Guerra-Ojeda, S., Vila, J. M., Aldasoro, M., Victor, V. M., & Mauricio, M. D. (2019). Targeting early atherosclerosis: A focus on oxidative stress and inflammation. Oxidative Medicine and Cellular Longevity, 2019, 8563845.
Wu, M. Y., Li, C. J., Hou, M. F., & Chu, P. Y. (2017). New Insights into the role of inflammation in the pathogenesis of atherosclerosis. International Journal of Molecular Sciences, 18, 2034.
Zhao, X., Chen, L., Wu, J., You, J., Hong, Q., & Ye, F. (2021). Transcription factor KLF15 inhibits the proliferation and migration of gastric cancer cells via regulating the TFAP2A-AS1/NISCH axis. Biology Direct, 16, 21.
Zhao, Y., Song, W., Wang, L., Rane, M. J., Han, F., & Cai, L. (2019). Multiple roles of KLF15 in the heart: Underlying mechanisms and therapeutic implications. Journal of Molecular and Cellular Cardiology, 129, 193–196.
Wang, X. P., Huang, Z., Li, Y. L., Jin, K. Y., Dong, D. J., Wang, J. X., & Zhao, X. F. (2022). Kruppel-like factor 15 integrated autophagy and gluconeogenesis to maintain glucose homeostasis under 20-hydroxyecdysone regulation. PLoS Genetics, 18, e1010229.
De Meyer, G. R., Grootaert, M. O., Michiels, C. F., et al. (2015). Autophagy in vascular disease. Circulation Research, 116, 468–479.
Zhang, H., Ge, S., Ni, B., He, K., Zhu, P., Wu, X., & Shao, Y. (2021). Augmenting ATG14 alleviates atherosclerosis and inhibits inflammation via promotion of autophagosome-lysosome fusion in macrophages. Autophagy, 17, 4218–4230.
Acknowledgements
Not applicable.
Funding
Scientific research project of Wuxi Municipal Health Commission (M202109).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
The present study was approved by the Affiliated hospital of Jiangnan University (Ethics Code: LS2021083).
Consent to Participate
All samples provided written informed consent.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, D., Huang, M., Chang, Z. et al. KLF15 Transcriptionally Activates ATG14 to Promote Autophagy and Attenuate Damage of ox-LDL-Induced HAECs. Mol Biotechnol 66, 112–122 (2024). https://doi.org/10.1007/s12033-023-00742-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-00742-x