Abstract
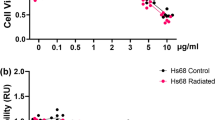
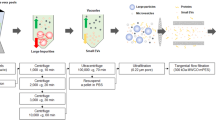
In the epidermal and dermal layers of the skin, diverse cell types are reconstituted during the wound healing process. Delays or failures in wound healing are a major issue in skin therapy because they prevent the normal structure and function of wounded tissue from being restored, resulting in ulceration or other skin abnormalities. Human immortalized keratinocytes (HaCAT) cells are a spontaneously immortalized human keratinocyte cell line capable of secreting many bioactive chemicals (a secretome) that stimulate skin cell proliferation, rejuvenation, and regeneration. In this study, the HaCaT secretome was encapsulated with polyesters such as poly (lactic-co-glycolic acid) (PLGA) and cassava starch in an effort to maximize its potential. According to the estimated mechanism of the HaCaT secretome, all treatments were conducted on immortalized dermal fibroblast cell lines, a model of wound healing. Encapsulation of HaCaT secretome and cassava starch enhanced the effectiveness of cell proliferation, migration, and anti-aging. On the other hand, the levels of reactive oxygen species (ROS) were lowered, activating antioxidants in immortalized dermal fibroblast cells. The HaCaT secretome induced in a dose-dependent manner the expression of antioxidant-associated genes, including SOD, CAT, and GPX. Six cytokines, including CCL2 and MCP-1, influenced immunoregulatory and inflammatory processes in cultured HaCAT cells. HaCaT secretome encapsulated in cassava starch can reduce ROS buildup by boosting antioxidant to stimulate wound healing. Hence, the HaCaT secretome may have a new chance in the cosmetics business to develop components for wound prevention and healing.





Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Oprea, T. I., Bologa, C. G., Brunak, S., Campbell, A., Gan, G. N., Gaulton, A., Gomez, S. M., Guha, R., Hersey, A., & Holmes, J. (2018). Unexplored therapeutic opportunities in the human genome. Nature Reviews Drug Discovery, 17(5), 317–332.
Johnson, D. E. (2018). Biotherapeutics: Challenges and opportunities for predictive toxicology of monoclonal antibodies. International Journal of Molecular Sciences, 19(11), 3685.
Lu, R.-M., Hwang, Y.-C., Liu, I.-J., Lee, C.-C., Tsai, H.-Z., Li, H.-J., & Wu, H.-C. (2020). Development of therapeutic antibodies for the treatment of diseases. Journal of Biomedical Science, 27(1), 1–30.
Aubert, G., & Lansdorp, P. M. (2008). Telomeres and aging. Physiological reviews, 88(2), 557–579.
Vizoso, F. J., Eiro, N., Cid, S., Schneider, J., & Perez-Fernandez, R. (2017). Mesenchymal stem cell secretome: Toward cell-free therapeutic strategies in regenerative medicine. International Journal of Molecular Sciences, 18(9), 1852.
Seo, M.-D., Kang, T. J., Lee, C. H., Lee, A.-Y., & Noh, M. (2012). HaCaT keratinocytes and primary epidermal keratinocytes have different transcriptional profiles of cornified envelope-associated genes to T helper cell cytokines. Biomolecules & Therapeutics, 20(2), 171.
Harding, C., Heuser, J., & Stahl, P. (1983). Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. The Journal of Cell Biology, 97(2), 329–339.
Yu, B., Zhang, X., & Li, X. (2014). Exosomes derived from mesenchymal stem cells. International Journal of Molecular Sciences, 15(3), 4142–4157.
Daneshi, N., Bahmaie, N., & Esmaeilzadeh, A. (2022). Cell-free treatments: a new generation of targeted therapies for treatment of ischemic heart diseases. Cell Journal (Yakhteh), 24(7), 353.
Damayanti, R. H., Rusdiana, T., & Wathoni, N. (2021). Mesenchymal stem cell secretome for dermatology application: A review. Clinical, Cosmetic and Investigational Dermatology, 14, 1401.
Shpichka, A., Butnaru, D., Bezrukov, E. A., Sukhanov, R. B., Atala, A., Burdukovskii, V., Zhang, Y., & Timashev, P. (2019). Skin tissue regeneration for burn injury. Stem Cell Research & Therapy, 10, 1–16.
Damayanti, R. H., Rusdiana, T., & Wathoni, N. (2021). Mesenchymal stem cell secretome for dermatology application: a review. Clinical, Cosmetic and Investigational Dermatology. https://doi.org/10.2147/CCID.S331044
Tan, K. X., Chang, T., & Lin, X. (2022). Secretomes as an emerging class of bioactive ingredients for enhanced cosmeceutical applications. Experimental Dermatology, 31(5), 674–688.
Bobadilla, A. V. P., Arévalo, J., Sarró, E., Byrne, H. M., Maini, P. K., Carraro, T., Balocco, S., Meseguer, A., & Alarcón, T. (2019). In vitro cell migration quantification method for scratch assays. Journal of the Royal Society Interface, 16(151), 20180709.
Li, B. (2011). Wang JH-C: Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. Journal of Tissue Viability, 20(4), 108–120.
Thapa, R. K., Margolis, D. J., Kiick, K. L., & Sullivan, M. O. (2020). Enhanced wound healing via collagen-turnover-driven transfer of PDGF-BB gene in a murine wound model. ACS Applied Bio Materials, 3(6), 3500–3517.
Yallapu, M. M., Gupta, B. K., Jaggi, M., & Chauhan, S. C. (2010). Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. Journal of Colloid and Interface Science, 351(1), 19–29.
Reddy, L. H. (2005). Drug delivery to tumours: Recent strategies. Journal of Pharmacy and Pharmacology, 57(10), 1231–1242.
Shi, A., Li, D., Liu, H., Adhikari, B., & Wang, Q. (2016). Effect of drying and loading methods on the release behavior of ciprofloxacin from starch nanoparticles. International Journal of Biological Macromolecules, 87, 55–61.
Xie, X., Zhang, Y., Zhu, Y., & Lan, Y. (2022). Preparation and drug-loading properties of amphoteric cassava starch nanoparticles. Nanomaterials, 12(4), 598.
Gensure, R. C., Mäkitie, O., Barclay, C., Chan, C., DePalma, S. R., Bastepe, M., Abuzahra, H., Couper, R., Mundlos, S., & Sillence, D. (2005). A novel COL1A1 mutation in infantile cortical hyperostosis (Caffey disease) expands the spectrum of collagen-related disorders. The Journal of Clinical Investigation, 115(5), 1250–1257.
Pham-Huy, L. A., He, H., & Pham-Huy, C. (2008). Free radicals, antioxidants in disease and health. International Journal of Biomedical Science: IJBS, 4(2), 89.
Singh, S., Anshita, D., & Ravichandiran, V. (2021). MCP-1: Function, regulation, and involvement in disease. International Immunopharmacology, 101, 107598.
Korbecki, J., Barczak, K., Gutowska, I., Chlubek, D., & Baranowska-Bosiacka, I. (2022). CXCL1: Gene, promoter, regulation of expression, mRNA stability, regulation of activity in the intercellular space. International Journal of Molecular Sciences, 23(2), 792.
Iso, Y., Spees, J. L., Serrano, C., Bakondi, B., Pochampally, R., Song, Y.-H., Sobel, B. E., Delafontaine, P., & Prockop, D. J. (2007). Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochemical and Biophysical Research Communications, 354(3), 700–706.
Du, L., Lv, R., Yang, X., Cheng, S., Ma, T., & Xu, J. (2016). Hypoxic conditioned medium of placenta-derived mesenchymal stem cells protects against scar formation. Life Sciences, 149, 51–57.
Ahangar, P., Mills, S. J., Smith, L. E., Strudwick, X. L., Ting, A. E., Vaes, B., & Cowin, A. J. (2020). Human multipotent adult progenitor cell-conditioned medium improves wound healing through modulating inflammation and angiogenesis in mice. Stem Cell Research & Therapy, 11(1), 1–16.
Saheli, M., Bayat, M., Ganji, R., Hendudari, F., Kheirjou, R., Pakzad, M., Najar, B., & Piryaei, A. (2020). Human mesenchymal stem cells-conditioned medium improves diabetic wound healing mainly through modulating fibroblast behaviors. Archives of Dermatological Research, 312, 325–336.
Hu, Q., Luni, C., & Elvassore, N. (2018). Microfluidics for secretome analysis under enhanced endogenous signaling. Biochemical and Biophysical Research Communications, 497(2), 480–484.
Song, X., Zhao, Y., Hou, S., Xu, F., Zhao, R., He, J., Cai, Z., Li, Y., & Chen, Q. (2008). Dual agents loaded PLGA nanoparticles: Systematic study of particle size and drug entrapment efficiency. European Journal of Pharmaceutics and Biopharmaceutics, 69(2), 445–453.
Le Corre, D., Bras, J., & Dufresne, A. (2010). Starch nanoparticles: A review. Biomacromolecules, 11(5), 1139–1153.
Ahmad, M., Gani, A., Hassan, I., Huang, Q., & Shabbir, H. (2020). Production and characterization of starch nanoparticles by mild alkali hydrolysis and ultra-sonication process. Scientific Reports, 10(1), 1–11.
King, A., Balaji, S., Le, L. D., Crombleholme, T. M., & Keswani, S. G. (2014). Regenerative wound healing: The role of interleukin-10. Advances in Wound Care, 3(4), 315–323.
Promjantuek, W., Chaicharoenaudomrung, N., Phonchai, R., Kunhorm, P., & Noisa, P. (2022). Transgenic immortalization of human dermal fibroblasts mediated through the microRNA/SIRT1 pathway. In Vivo, 36(1), 140–152.
Verhaegen, P. D., Van Zuijlen, P. P., Pennings, N. M., Van Marle, J., Niessen, F. B., Van Der Horst, C. M., & Middelkoop, E. (2009). Differences in collagen architecture between keloid, hypertrophic scar, normotrophic scar, and normal skin: An objective histopathological analysis. Wound Repair and Regeneration, 17(5), 649–656.
Xue, M., & Jackson, C. J. (2015). Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Advances in Wound Care, 4(3), 119–136.
Prochasson, P., Delouis, C., & Brison, O. (2002). Transcriptional deregulation of the keratin 18 gene in human colon carcinoma cells results from an altered acetylation mechanism. Nucleic Acids Research, 30(15), 3312–3322.
Butler, C., Sprowls, S., Szalai, G., Arsiwala, T., Saralkar, P., Straight, B., Hatcher, S., Tyree, E., Yost, M., & Kohler, W. J. (2020). Hypomethylating agent azacitidine is effective in treating brain metastasis triple-negative breast cancer through regulation of DNA methylation of keratin 18 gene. Translational Oncology, 13(6), 100775.
Munteanu, I. G., & Apetrei, C. (2021). Analytical methods used in determining antioxidant activity: A review. International Journal of Molecular Sciences, 22(7), 3380.
Akita, S., Akino, K., & Hirano, A. (2013). Basic fibroblast growth factor in scarless wound healing. Advances in wound care, 2(2), 44–49.
Zhu, S., Liu, M., Bennett, S., Wang, Z., Pfleger, K. D., & Xu, J. (2021). The molecular structure and role of CCL2 (MCP-1) and C-C chemokine receptor CCR2 in skeletal biology and diseases. Journal of Cellular Physiology, 236(10), 7211–7222.
Kunhorm, P., Chaicharoenaudomrung, N., & Noisa, P. (2023). Cordycepin-induced keratinocyte secretome promotes skin cell regeneration. In Vivo, 37(2), 574–590.
Kunz, M., & Ibrahim, S. M. (2009). Cytokines and cytokine profiles in human autoimmune diseases and animal models of autoimmunity. Mediators of Inflammation. https://doi.org/10.1155/2009/979258
Acknowledgements
This work was supported by Agricultural Research Development Agency (ARDA), Suranaree University of Technology (SUT), Thailand Science Research and Innovation (TSRI), and National Science, Research, and Innovation Fund (NSRF) (project code 90464).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heebkaew, N., Promjantuek, W., Chaicharoenaudomrung, N. et al. Encapsulation of HaCaT Secretome for Enhanced Wound Healing Capacity on Human Dermal Fibroblasts. Mol Biotechnol 66, 44–55 (2024). https://doi.org/10.1007/s12033-023-00732-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-00732-z