Abstract
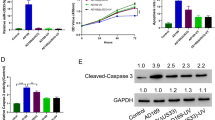
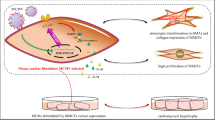
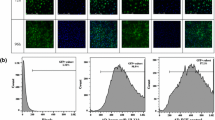
Our previous study demonstrated in vivo that mouse cytomegalovirus (MCMV) infection promoted vascular remodeling after downregulation of miR-1929-3p. This study aimed to investigate the role of miR-1929-3p/ETAR/NLRP3 pathway in mouse vascular smooth muscle cells (MOVAS) after MCMV infection. First, PCR was used to detect the success of the infection. Second, MOVAS were transfected with the miR-1929-3p mimic, inhibitor, and ETAR overexpressed adenovirus vector. Cell proliferation was detected using EdU, whereas apoptosis was detected using flow cytometry. The expression of miR-1929-3p and ETAR were detected using qRT-PCR. Western blot detected proteins of cell proliferation, apoptosis, and the NLRP3 inflammasome. Interleukin-1β and interleukin-18 were determined using ELISA. The results revealed that after 48 h, MCMV infection promoted the proliferation of MOVAS when the MOI was 0.01. MCMV infection increased ETAR by downregulating miR-1929-3p. The miR-1929-3p mimic reversed the proliferation and apoptosis, whereas the miR-1929-3p inhibitor promoted this effect. ETAR overexpression further promoted MCMV infection by downregulating miR-1929-3p-mediated proliferation and apoptosis. MCMV infection mediates the downregulation of miR-1929-3p and the upregulation of ETAR, which activates NLRP3 inflammasome. In conclusion, MCMV infection promoted the proliferation of MOVAS, possibly by downregulating miR-1929-3p, promoting the upregulation of the target gene ETAR and activating NLRP3 inflammasome.








Similar content being viewed by others
Abbreviations
- MCMV:
-
Mouse cytomegalovirus
- MOVAS:
-
Mouse vascular smooth muscle cells
- VSMCs:
-
Vascular smooth muscle cells
- miRNA:
-
MicroRNA
- ETAR:
-
Endothelin receptor type A
References
Zahedmehr, A.(2018). Chapter 17-Hypertension. Practical Cardiology. St. Louis, Missouri: Eds. 291–302
Owens, G. K., Kumar, M. S., & Wamhoff, B. R. (2004). Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiological Reviews., 84, 767–801.
Lacolley, P., Regnault, V., Nicoletti, A., Li, Z., & Michel, J.-B. (2012). The vascular smooth muscle cell in arterial pathology: A cell that can take on multiple roles. Cardiovascular Research., 95, 194–204.
Feihl, F., Liaudet, L., Levy, B. I., & Waeber, B. (2008). Hypertension and microvascular remodelling. Cardiovascular Research, 78, 274–285.
Landolfo, S., Gariglio, M., Gribaudo, G., & Lembo, D. (2003). The human cytomegalovirus. Pharmacology & Therapeutics, 98, 269–297.
Lu, T. X., & Rothenberg, M. E. (2018). MicroRNA. The Journal of Allergy and Clinical Immunology, 141, 1202–1207.
Stark, T. J., Arnold, J. D., Spector, D. H., & Yeo, G. W. (2012). High-resolution profiling and analysis of viral and host small RNAs during human cytomegalovirus infection. Journal of Virology, 86, 226–235.
Dhuruvasan, K., Sivasubramanian, G., & Pellett, P. E. (2011). Roles of host and viral microRNAs in human cytomegalovirus biology. Virus Research, 157, 180–192.
Ng, K. R., Li, J. Y. Z., & Gleadle, J. M. (2015). Human cytomegalovirus encoded microRNAs: Hitting targets. Expert Review of Anti-Infective Therapy., 13, 1469–1479.
Serneri, G. G., Cecioni, I., Vanni, S., Paniccia, R., Bandinelli, B., Vetere, A., Janming, X., Bertolozzi, I., Boddi, M., Lisi, G. F., Sani, G., & Modesti, P. A. (2000). Selective upregulation of cardiac endothelin system in patients with ischemic but not idiopathic dilated cardiomyopathy: Endothelin-1 system in the human failing heart. Circulation Research, 86, 377–385.
Southwood, M., Ross, R. V. M., Kuc, R. E., Hagan, G., Sheares, K. K., Jenkins, D. P., Goddard, M., Davenport, A. P., & Pepke-Zaba, J. (2016). Endothelin ETA receptors predominate in chronic thromboembolic pulmonary hypertension. Life Sciences, 159, 104–110.
Amiri, F., Virdis, A., Neves, M. F., Iglarz, M., Seidah, N. G., Touyz, R. M., Reudelhuber, T. L., & Schiffrin, E. L. (2004). Endothelium-restricted overexpression of human endothelin-1 causes vascular remodeling and endothelial dysfunction. Circulation, 110, 2233–2240.
Jern, S. (1992). Pathophysiology of cardiovascular structural changes in hypertension. Clinical and experimental hypertension. Part A, Theory and practice., 14, 163–172.
Agapitov, A. V., & Haynes, W. G. (2002). Role of endothelin in cardiovascular disease. Journal of the renin-angiotensin-aldosterone system: JRAAS, 3, 1–15.
Vanêcková, I., Kramer, H. J., Bäcker, A., Vernerová, Z., Opocensky, M., & Cervenka, L. (2005). Early endothelin—A receptor blockade decreases blood pressure and ameliorates end-organ damage in homozygous Ren-2 rats. Hypertension (Dalls, Tex.: 1979), 46, 969–974.
Sutterwala, F. S., Haasken, S., & Cassel, S. L. (2014). Mechanism of NLRP3 inflammasome activation. Annals of the New York Academy of Sciences, 1319, 82–95.
Kelley, N., Jeltema, D., Duan, Y., & He, Y. (2019). The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. International Journal of Molecular Sciences, 20(13), 3328.
Wang, J., Wu, Q., Yu, J., Cao, X., & Xu, Z. (2019). miR-125a-5p inhibits the expression of NLRP3 by targeting CCL4 in human vascular smooth muscle cells treated with ox-LDL. Experimental and Therapeutic Medicine, 18, 1645–1652.
Zhou, W., Xi, D., Shi, Y., Wang, L., Zhong, H., Huang, Z., Liu, Y., Tang, Y., Lu, N., Wang, Y., Zhang, Z., Pei, J., Tang, N., & He, F. (2021). MicroRNA-1929-3p participates in murine cytomegalovirus-induced hypertensive vascular remodeling through Ednra/NLRP3 inflammasome activation. International Journal of Molecular Medicine, 47, 719–731.
Li, P., Zhu, N., Yi, B., Wang, N., Chen, M., You, X., Zhao, X., Solomides, C. C., Qin, Y., & Sun, J. (2013). MicroRNA-663 regulates human vascular smooth muscle cell phenotypic switch and vascular neointimal formation. Circulation Research, 113, 1117–1127.
Kang, H., & Hata, A. (2012). MicroRNA regulation of smooth muscle gene expression and phenotype. Current Opinion in Hematology, 19, 224–231.
Popović, M., Smiljanić, K., Dobutović, B., Syrovets, T., Simmet, T., & Isenović, E. R. (2012). Human cytomegalovirus infection and atherothrombosis. Journal of Thrombosis and Thrombolysis, 33, 160–172.
Hummel, M., Zhang, Z., Yan, S., DePlaen, I., Golia, P., Varghese, T., Thomas, G., & Abecassis, M. I. (2001). Allogeneic transplantation induces expression of cytomegalovirus immediate-early genes in vivo: A model for reactivation from latency. Journal of Virology, 75, 4814–4822.
Shi, Y., Xi, D., Zhang, X., Huang, Z., Tang, N., Liu, Y., Wang, L., Tang, Y., Zhong, H., & He, F. (2020). Screening and validation of differentially expressed microRNAs and target genes in hypertensive mice induced by cytomegalovirus infection. Bioscience Reports, 40(12).
Li, X., Wei, Y., & Wang, Z. (2018). microRNA-21 and hypertension. Hypertension research: Official journal of the Japanese Society of Hypertension, 41, 649–661.
Chistiakov, D. A., Sobenin, I. A., Orekhov, A. N., & Bobryshev, Y. V. (2015). Human miR-221/222 in physiological and atherosclerotic vascular remodeling. BioMed Research International, 354517.
Thyberg, J. (1998). Phenotypic modulation of smooth muscle cells during formation of neointimal thickenings following vascular injury. Histology and Histopathology, 13, 871–891.
Nemenoff, R. A., Horita, H., Ostriker, A. C., Furgeson, S. B., Simpson, P. A., VanPutten, V., Crossno, J., Offermanns, S., & Weiser-Evans, M. C. M. (2011). SDF-1α induction in mature smooth muscle cells by inactivation of PTEN is a critical mediator of exacerbated injury-induced neointima formation. Arteriosclerosis, Thrombosis, and Vascular Biology, 31, 1300–1308.
Touyz, R. M., Alves-Lopes, R., Rios, F. J., Camargo, L. L., Anagnostopoulou, A., Arner, A., & Montezano, A. C. (2018). Vascular smooth muscle contraction in hypertension. Cardiovascular Research, 114, 529–539.
Gurbanov, E., & Shiliang, X. (2006). The key role of apoptosis in the pathogenesis and treatment of pulmonary hypertension. European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for Cardio-thoracic Surgery, 30, 499–507.
Kockx, M. M., & Herman, A. G. (2000). Apoptosis in atherosclerosis: Beneficial or detrimental? Cardiovascular Research, 45, 736–746.
Schrijvers, D. M., Meyer, G. R. Y. D., Kockx, M. M., Herman, A. G., & Martinet, W. (2005). Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology, 25, 1256–1261.
Schneider, M. P., Boesen, E. I., & Pollock, D. M. (2007). Contrasting actions of endothelin ET(A) and ET(B) receptors in cardiovascular disease. Annual Review of Pharmacology and Toxicology, 47, 731–759.
Zhao, C., & Zhao, W. (2020). NLRP3 Inflammasome—a key player in antiviral responses. Frontiers in Immunology, 11, 211.
Sun, H.-J., Ren, X.-S., Xiong, X.-Q., Chen, Y.-Z., Zhao, M.-X., Wang, J.-J., Zhou, Y.-B., Han, Y., Chen, Q., Li, Y.-H., Kang, Y.-M., & Zhu, G.-Q. (2017). NLRP3 inflammasome activation contributes to VSMC phenotypic transformation and proliferation in hypertension. Cell Death & Disease, 8, e3074.
Krishnan, S. M., Sobey, C. G., Latz, E., Mansell, A., & Drummond, G. R. (2014). IL-1β and IL-18: Inflammatory markers or mediators of hypertension? British Journal of Pharmacology, 171, 5589–5602.
Schmidt, R. L., & Lenz, L. L. (2012). Distinct licensing of IL-18 and IL-1β secretion in response to NLRP3 inflammasome activation. PloS One, 7, e45186.
Pirhonen, J., Sareneva, T., Kurimoto, M., Julkunen, I., & Matikainen, S. (1999). Virus infection activates IL-1 beta and IL-18 production in human macrophages by a caspase-1-dependent pathway. Journal of Immunology (Baltimore, Md.: 1950), 162, 7322–7329.
Loomis, E. D., Sullivan, J. C., Osmond, D. A., Pollock, D. M., & Pollock, J. S. (2005). Endothelin mediates superoxide production and vasoconstriction through activation of NADPH oxidase and uncoupled nitric-oxide synthase in the rat aorta. The Journal of Pharmacology and Experimental Therapeutics, 315, 1058–1064.
Vajjhala, P. R., Mirams, R. E., & Hill, J. M. (2012). Multiple binding sites on the pyrin domain of ASC protein allow self-association and interaction with NLRP3 protein. The Journal of Biological Chemistry, 287, 41732–41743.
Duncan, J. A., Bergstralh, D. T., Wang, Y., Willingham, S. B., Ye, Z., Zimmermann, A. G., & Ting, J.P.-Y. (2007). Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proceedings of the National Academy of Sciences of the United States of America., 104, 8041–8046.
Coll, R. C., Hill, J. R., Day, C. J., Zamoshnikova, A., Boucher, D., Massey, N. L., Chitty, J. L., Fraser, J. A., Jennings, M. P., Robertson, A. A. B., & Schroder, K. (2019). MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nature Chemical Biology, 15, 556.
Funding
The present study was supported by the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (Grant No. 2020-PT330-003), the grants from the National Natural Science Foundation of China (Grant No. 31960187), the National College Students innovation and Entrepreneurship training program (Grant No. 202010759027), the Scientific research project of Shihezi University (Grant No. ZZZC201921A), the “3152” project of Shihezi University (Grant No. ZG010308), and the Youth Innovation and Talent Cultivation project of Shihezi University (grant NO.CXPY202216).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Ethical Approval
All animal studies were performed according to the guidelines of the Chinese Council on Animal Care, and this study was approved by the Ethics Committee of Shihezi Medical University (Shihezi, China).
Informed Consent
From all participants whose personal information is contained in this manuscript the additional written voluntary consent was obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, L., Zhou, W., Wang, L. et al. Murine Cytomegalovirus Infection Induced miR-1929-3p Down-Regulation Promotes the Proliferation and Apoptosis of Vascular Smooth Muscle Cells in Mice by Targeting Endothelin A Receptor and Downstream NLRP3 Activation Pathway. Mol Biotechnol 65, 1954–1967 (2023). https://doi.org/10.1007/s12033-023-00720-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-00720-3