Abstract
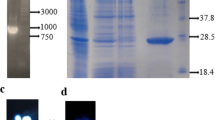
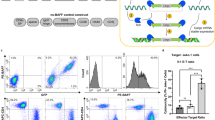
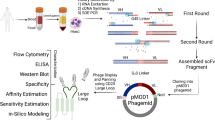
As a member of the tumor necrosis factor (TNF) superfamily, the B-cell activating factor (BAFF) plays a crucial role in B-cell survival and differentiation. Overexpression of this protein has been closely linked to autoimmune disorders and some B-cell malignancies. Using monoclonal antibodies (mAbs) against the BAFF soluble domain appears to be a complementary treatment for some of these diseases. This study aimed to produce and develop a specific Nanobody (Nb), a variable camelid antibody domain, against the soluble domain of BAFF protein. After camel immunization with recombinant protein and preparing cDNA from total RNAs separated from camel lymphocytes, an Nb library was developed. Individual colonies capable of binding selectively to rBAFF were obtained by periplasmic-ELISA, sequenced, and expressed in a bacterial expression system. The specificity and affinity of selected Nb were determined and its target identification and functionality were evaluated using flow cytometry.











Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Smith, D., & Germolec, D. (1999). Introduction to immunology and autoimmunity. Enviromental Health Perspectives, 107(Suppl. 5), 661–665. https://doi.org/10.1289/ehp.99107s5661
Singh, S. P., Wal, P., Wal, A., Srivastava, V., Tiwari, R., & Sharma, R. D. (2016). Understanding autoimmune disease: An update review. IJPTB, 3, 51–65.
Wang, L., Wang, F. S., & Gershwin, M. E. (2015). Human autoimmune diseases: A comprehensive update. Journal of Internal Medicine, 278(4), 369–395.
Köhler, G., & Milstein, C. (1975). Continuous cultures of fused cells secreting antibody of predefined specificity. Nature, 256(5517), 495–7.
Racadot, E., Wendling, D., Rumbach, L., Wijdenes, J., & Herve, P. (1994). Current concepts in the treatment of autoimmune diseases with monoclonal antibodies. Clinical Immunotherapeutics, 1, 199–208.
Jamilloux, Y., El Jammal, T., Vuitton, L., Gerfaud-Valentin, M., Kerever, S., & Sève, P. (2019). JAK inhibitors for the treatment of autoimmune and inflammatory diseases. Autoimmunity Reviews, 18(11), 102390.
Mackay, F., & Browning, J. L. (2002). BAFF: A fundamental survival factor for B cells. Nature Reviews Immunology, 2(7), 465–475.
Vincent, F. B., Morand, E. F., Schneider, P., & Mackay, F. (2014). The BAFF/APRIL system in SLE pathogenesis. Nature Reviews Rheumatology, 10(6), 365–373.
Schneider, P., MacKay, F., Steiner, V., Hofmann, K., Bodmer, J.-L., Holler, N., et al. (1999). BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. The Journal of Experimental Medicine, 189(11), 1747–1756.
Davidson, A. (2010). Targeting BAFF in autoimmunity. Current Opinion in Immunology, 22(6), 732–739.
Sakurai, D., Kanno, Y., Hase, H., Kojima, H., Okumura, K., & Kobata, T. (2007). TACI attenuates antibody production costimulated by BAFF-R and CD40. European Journal of Immunology, 37(1), 110–118.
Stadanlick, J. E., Kaileh, M., Karnell, F. G., Scholz, J. L., Miller, J. P., Quinn Iii, W. J., et al. (2008). Tonic B cell antigen receptor signals supply an NF-κB substrate for prosurvival BLyS signaling. Nature Immunology, 9(12), 1379–1387.
Rickert, R. C., Jellusova, J., & Miletic, A. V. (2011). Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunological Reviews, 244(1), 115–133.
Mackay, F., & Schneider, P. (2008). TACI, an enigmatic BAFF/APRIL receptor, with new unappreciated biochemical and biological properties. Cytokine & Growth Factor Reviews, 19(3–4), 263–276.
Hengeveld, P., & Kersten, M. (2015). B-cell activating factor in the pathophysiology of multiple myeloma: a target for therapy? Blood Cancer Journal, 5(2), e282-e.
Rihacek, M., Bienertova-Vasku, J., Valik, D., Sterba, J., Pilatova, K., & Zdrazilova-Dubska, L. (2015). B-cell activating factor as a cancer biomarker and its implications in cancer-related cachexia. BioMed Research International. https://doi.org/10.1155/2015/792187
Manetta, J., Bina, H., Ryan, P., Fox, N., Witcher, D. R., & Kikly, K. (2014). Generation and characterization of tabalumab, a human monoclonal antibody that neutralizes both soluble and membrane-bound B-cell activating factor. Journal of inflammation research, 7, 121.
Liu, Z., & Davidson, A. (2011). BAFF inhibition: A new class of drugs for the treatment of autoimmunity. Experimental Cell Research, 317(9), 1270–1277.
Chan, A. C., & Carter, P. J. (2010). Therapeutic antibodies for autoimmunity and inflammation. Nature Reviews Immunology, 10(5), 301–316.
Singh, S., Tank, N. K., Dwiwedi, P., Charan, J., Kaur, R., Sidhu, P., et al. (2018). Monoclonal antibodies: A review. Current Clinical Pharmacology, 13(2), 85–99.
Giritch, A., Marillonnet, S., Engler, C., van Eldik, G., Botterman, J., Klimyuk, V., et al. (2006). Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. Proceedings of the National Academy of Sciences, 103(40), 14701–14706.
Kholodenko, R. V., Kalinovsky, D. V., Doronin, I. I., Ponomarev, E. D., & Kholodenko, I. V. (2019). Antibody fragments as potential biopharmaceuticals for cancer therapy: Success and limitations. Current Medicinal Chemistry, 26(3), 396–426.
Doyle, P. J., Arbabi-Ghahroudi, M., Gaudette, N., Furzer, G., Savard, M. E., Gleddie, S., et al. (2008). Cloning, expression, and characterization of a single-domain antibody fragment with affinity for 15-acetyl-deoxynivalenol. Molecular Immunology, 45(14), 3703–3713.
Pardon, E., Laeremans, T., Triest, S., Rasmussen, S. G., Wohlkönig, A., Ruf, A., et al. (2014). A general protocol for the generation of Nanobodies for structural biology. Nature Protocols, 9(3), 674–693.
Jovčevska, I., Zupanec, N., Kočevar, N., Cesselli, D., Podergajs, N., Stokin, C. L., et al. (2014). TRIM28 and β-actin identified via nanobody-based reverse proteomics approach as possible human glioblastoma biomarkers. PLoS ONE, 9(11), e113688.
Movahedi, K., Schoonooghe, S., Laoui, D., Houbracken, I., Waelput, W., Breckpot, K., et al. (2012). Nanobody-based targeting of the macrophage mannose receptor for effective in vivo imaging of tumor-associated macrophages. Cancer Research, 72(16), 4165–4177.
Ranjibar, F., Habibi-Anbouhi, M., Kazemi-Lomedasht, F., Aghaee-Bakhtiyari, S. H., Alirahimi, E., & Behdani, M. (2018). Cell-specific targeting by engineered M13 bacteriophage expressing VEGFR2 nanobody. Iranian Journal of Basic Medical Sciences, 21(9), 884.
Martineau, P. (2010). Affinity measurements by competition ELISA (pp. 657–665). Springer.
Beatty, J. D., Beatty, B. G., & Vlahos, W. G. (1987). Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. Journal of Immunological Methods, 100(1–2), 173–179.
Hsu, B. L., Harless, S. M., Lindsley, R. C., Hilbert, D. M., & Cancro, M. P. (2002). Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. The Journal of Immunology, 168(12), 5993–5996.
Baker, K. P., Edwards, B. M., Main, S. H., Choi, G. H., Wager, R. E., Halpern, W. G., et al. (2003). Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis & Rheumatism, 48(11), 3253–3265.
Furie, R., Stohl, W., Ginzler, E. M., Becker, M., Mishra, N., Chatham, W., et al. (2008). Biologic activity and safety of belimumab, a neutralizing anti-B-lymphocyte stimulator (BLyS) monoclonal antibody: A phase I trial in patients with systemic lupus erythematosus. Arthritis Research & Therapy, 10(5), 1–15.
Zhang, F., Bae, S.-C., Bass, D., Chu, M., Egginton, S., Gordon, D., et al. (2018). A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Annals of the Rheumatic Diseases, 77(3), 355–363.
Van Vollenhoven, R., Kinnman, N., Vincent, E., Wax, S., & Bathon, J. (2011). Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: Results of a phase II, randomized, placebo-controlled trial. Arthritis & Rheumatism, 63(7), 1782–1792.
Bruno, V., Battaglia, G., & Nicoletti, F. (2011). The advent of monoclonal antibodies in the treatment of chronic autoimmune diseases. Neurological Sciences, 31(3), 283–288.
Furie, R., Petri, M., Zamani, O., Cervera, R., Wallace, D. J., Tegzová, D., et al. (2011). A phase 3, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits BLyS, in patients with systemic lupus erythematosus. Arthritis and Rheumatism, 63(12), 3918.
Levy, R. A., Gonzalez-Rivera, T., Khamashta, M., Fox, N. L., Jones-Leone, A., Rubin, B., et al. (2021). 10 Years of belimumab experience: What have we learnt? Lupus, 30(11), 1705–1721.
Hamers-Casterman, C., Atarhouch, T., Muyldermans, Sa., Robinson, G., Hammers, C., Songa, E. B., et al. (1993). Naturally occurring antibodies devoid of light chains. Nature, 363(6428), 446–8.
Moghimi, S. M., Rahbarizadeh, F., Ahmadvand, D., & Parhamifar, L. (2013). Heavy chain only antibodies: a new paradigm in personalized HER2+ breast cancer therapy. BioImpacts, 3(1), 1.
Cortez-Retamozo, V., Lauwereys, M., Hassanzadeh, Gh. G., Gobert, M., Conrath, K., Muyldermans, S., et al. (2002). Efficient tumor targeting by single-domain antibody fragments of camels. International Journal of Cancer, 98(3), 456–462.
Hussack, G., Hirama, T., Ding, W., MacKenzie, R., & Tanha, J. (2011). Engineered single-domain antibodies with high protease resistance and thermal stability. PLoS ONE, 6(11), e28218.
Dumoulin, M., Conrath, K., Van Meirhaeghe, A., Meersman, F., Heremans, K., Frenken, L. G., et al. (2002). Single-domain antibody fragments with high conformational stability. Protein Science, 11(3), 500–515.
Mir, M. A., Mehraj, U., Sheikh, B. A., & Hamdani, S. S. (2020). Nanobodies: The “magic bullets” in therapeutics, drug delivery and diagnostics. Human Antibodies, 28(1), 29–51.
Nie, S., Wang, Z., Moscoso-Castro, M., D’Souza, P., Lei, C., Xu, J., et al. (2020). Biology drives the discovery of bispecific antibodies as innovative therapeutics. Antibody Therapeutics, 3(1), 18–62.
Yang, E. Y., & Shah, K. (2020). Nanobodies: Next generation of cancer diagnostics and therapeutics. Frontiers in Oncology, 10, 1182.
Jindal, V., Khoury, J., Gupta, R., & Jaiyesimi, I. (2020). Current status of chimeric antigen receptor T-cell therapy in multiple myeloma. American Journal of Clinical Oncology, 43(5), 371–377.
Li, S., Jiang, K., Wang, T., Zhang, W., Shi, M., Chen, B., et al. (2020). Nanobody against PDL1. Biotechnology letters, 42(5), 727–736.
Kazemi-Lomedasht, F., Behdani, M., Habibi-Anbouhi, M., & Shahbazzadeh, D. (2016). Production and characterization of novel camel single domain antibody targeting mouse vascular endothelial growth factor. Monoclonal Antibodies in Immunodiagnosis and Immunotherapy, 35(3), 167–171.
Acknowledgements
This study was financially supported by Pasteur Institute of Iran (Grant Number 994) and the National Institute for Medical Research Development (NIMAD), grant no. 943314.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mardani-Jouneghani, R., Irani, S., Habibi-Anbouhi, M. et al. Development and Characterization of a Novel Single-Chain Antibody Against B-Cell Activating Factor. Mol Biotechnol 65, 1968–1978 (2023). https://doi.org/10.1007/s12033-023-00700-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-00700-7