Abstract
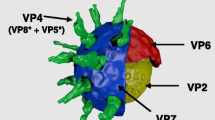
Group A rotavirus causes acute gastroenteritis in young ones of animals worldwide and is responsible for a high rate of their morbidity and mortality leading to huge economic losses. Developing affordable and safer vaccine on large scale is imperative to reach cattle population worldwide for the long-term control of diarrhea. Rotavirus middle capsid protein layer, VP6, is the most immunogenic and highly conserved protein that induces immune responses against rotavirus. In the present study, bovine group A rotavirus VP6 protein has been expressed for the first time in a highly nutritious and palatable forage crop, Trifolium alexandrinum, using Agrobacterium tumefaciens-mediated stable nuclear transformation. Transgenic nature of the shoots regenerated from cotyledon explants and rooted on hygromycin-containing medium was confirmed by polymerase chain reaction (PCR), Southern blot hybridization, reverse transcription-PCR (RT-PCR) and quantitative real-time PCR (qPCR), and protein expression and quantification by Western blot and enzyme-linked immune-sorbent assay (ELISA), respectively. The transformation efficiency of 2.10% was obtained. The highest amount of VP6 protein produced in a transgenic line was 402 ng/g fresh weights (0.03% of total soluble protein). Oral feeding of transgenic leafy shoots expressing VP6 protein stimulated systemic immunity by inducing significantly higher titers of anti-VP6 serum IgG antibodies in rabbit to reduce rotavirus infection. These transgenic fodder plants offer safer vaccine produced on large scale at low cost with reduced regulatory issues to improve livestock’s health and wealth. These plants would be used as alternative to the current live attenuated vaccines to protect young calves against rotavirus infection.






Similar content being viewed by others
Data Availability
The data obtained or analyzed in the present study has been incorporated in this manuscript.
References
Troeger, C., Khalil, I. A., Rao, P. C., Cao, S., Blacker, B. F., Ahmed, T., Armah, G., Bines, J. E., Brewer, T. G., & Colombara, D. V. (2018). Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 Years. JAMA Pediatrics, 172, 958–965.
Malik, Y. S., Kumar, N., Sharma, K., & Sharma, R. (2013). Epidemiology and genetic diversity of rotavirus strains associated with acute gastroenteritis in bovine, porcine, poultry and human population of Madhya Pradesh, Central India, 2004–2008. Advances in Animal Veterinary Sciences, 1, 111–115.
Estes, M. K., & Greenberg, H. B. (2013). Rotaviruses. In Fields Virology D. M. Knipe, & P. M. Howley (Eds.), Wolters Kluwer/Lippincott Williams and Wilkins Health (6th ed., pp 1347–1401). Philadelphia, PA
Steger, C. L., Boudreaux, C. E., LaConte, L. E., Pease, J. B., & McDonald, S. M. (2019). Group A rotavirus VP1 polymerase and VP2 core shell proteins: Intergenotypic sequence variation and in vitro functional compatibility. Journal of Virology, 93, e01642-e1718.
Witte, D., Handley, A., Jere, K. C., Bogandovic-Sakran, N., Mpakiza, A., Turner, A., Pavlic, D., Boniface, K., Mandolo, J., Suryawijaya, D., Bonnici, R., Justice, F., Bar-Zeev, N., Iturriza-Gomara, M., Ackland, J., Donato, C. M., Cowley, D., Barnes, G., Cunliffe, N. A., & Bines, J. E. (2022). Neonatal rotavirus vaccine (RV3-BB) immunogenicity and safety in a neonatal and infant administration schedule in Malawi: A randomised, double-blind, four-arm parallel group dose-ranging study. The Lancet Infectious Diseases, 22, 668–678.
Glass, R. I., Tate, J. E., Jiang, B., & Parashar, U. (2021). The rotavirus vaccine story: From discovery to the eventual control of rotavirus disease. Journal of Infectious Diseases, 224, S331–S342.
Vetter, V., Pereira, P., & Benninghoff, B. (2021). Rotavirus vaccination and intussusception: A paradigm shift? Human Vaccin Immunother, 17, 278–282.
Afchangi, A., Jalilvand, S., Mohajel, N., Marashi, S. M., & Shoja, Z. (2019). Rotavirus VP6 as a potential vaccine candidate. Reviews Medical Virology, 29, e2027.
Choi, A.H.-C., Basu, M., & McNeal, M. M. (2000). Functional mapping of protective domains and epitopes in the rotavirus VP6 protein. Journal of Virology, 74, 11574–11580.
Esquivel, F. R., Lopez, S., Guitierrez, X. L., & Arias, C. (2000). The internal rotavirus protein VP6 primes for an enhanced neutralizing antibody response. Archives of Virology, 145, 813–825.
Temprana, C. F., Arguelles, M. H., Gutierrez, N. M., Barril, P. A., Esteban, L. E., Silvestre, D., Mandile, M. G., Glikmann, G., & Castello, A. A. (2018). Rotavirus VP6 protein mucosally delivered by cell wall-derived particles from Lactococcus lactis induces protection against infection in a murine model. PLoS One, 13, e0203700.
Gonzalez, D. D., Mozgovoj, M. V., Bellido, D., Rodriguez, D. V., Fernandez, F. M., Wigdorovitz, A., Parreño, V. G., & Dus Santos, M. J. (2010). Evaluation of a bovine rotavirus VP6 vaccine efficacy in the calf model of infection and disease. Veterinary Immunology and Immunopathology, 137, 155–160.
Matsumura, T., Itchoda, N., & Tsunemitsu, H. (2002). Production of immunogenic VP6 protein of bovine group A rotavirus in transgenic potato plants. Archives of Virology, 147, 1263–1270.
Dong, J. L., Liang, B. G., Jin, Y. S., Zhang, W. J., & Wang, T. (2005). Oral immunization with pBsVP6-transgenic alfalfa protects mice against rotavirus infection. Virology, 339, 153–163.
Saldana, S., Esquivel, G. F., Olivera Flores, T. J., Arias, N., Lopez, S., Arias, C., Ruiz-Medrano, R., Mason, H., Mor, T., Richter, L., Arntzen, C. J., & Gomez Lim, M. A. (2006). Production of rotavirus-like particles in tomato (Lycopersicon esculentum L.) fruit by expression of capsid proteins VP2 and VP6 and immunological studies. Viral Immunology, 19, 42–53.
Feng, H., Li, X., Song, W., Duan, M., Chen, H., Wang, T., & Dong, J. (2017). Oral administration of a seed-based bivalent rotavirus vaccine containing VP6 and NSP4 induces specific immune responses in mice. Frontiers in Plant Science, 8, 910.
Borchers Inka, A. M., Gonzalez-Rabade, N., & Gray, J. C. (2012). Increased accumulation and stability of rotavirus VP6 protein in tobacco chloroplasts following changes to the 5′ untranslated region and the 5′ end of the coding region. Plant Biotechnology Journal, 10, 422–434.
Pêra, F. F. P. G., Mutepfa, D. L. R., & Khan, A. M. (2015). Engineering and expression of a human rotavirus candidate vaccine in Nicotiana benthamiana. Virology Journal, 12, 205.
Sanawati, A., Barlian, A., & Tan, M. I. (2017). Construction, in silico analysis, and in vitro expression of DNA vaccine candidate encoding human rotavirus VP6 capsid. International Journal of Integrative Biology, 18, 1–6.
Pastor, A. R., Rodriguez-Limas, W. A., Contreras, M. A., Esquivel, E., Esquivel-Guadarrama, F., Ramirez, O. T., & Palomares, L. A. (2014). The assembly conformation of rotavirus VP6 determines its protective efficacy against rotavirus challenge in mice. Vaccine, 32, 2874–2877.
Kurokawa, N., Lavoie, P.-O., D’Aoust, M.-A., Couture, M.M.-J., Dargis, M., Trépanier, S., Hoshino, S., Koike, T., Arai, M., & Tsutsui, N. (2021). Development and characterization of a plant-derived rotavirus-like particle vaccine. Vaccine, 39, 4979–4987. https://doi.org/10.1016/j.vaccine.2021.07.039
Lobato Gomez, M., Huang, X., Alvarez, D., He, W., Baysal, C., Zhu, C., Armario-Najera, V., et al. (2021). Contributions of the international plant science community to the fight against human infectious diseases-part 1: Epidemic and pandemic diseases. Plant Biotechnology Journal, 19, 1901–1920. https://doi.org/10.1111/pbi.13657
Bhoria, S., Yadav, J., Yadav, H., Chaudhary, D., Jaiwal, R., & Jaiwal, P. K. (2022). Current advances and future prospects in production of recombinant insulin and other proteins to treat diabetes mellitus. Biotechnology Letters, 44, 643–669.
Muhammad, D., Misri, B., EL-Nahrawy, M., Khan, S. & Serkan, A. (2014) Egyptian clover (Trifolium alexandrinum) FAO. Regional Office for the Near East and North Africa, Cairo
Abogadallah, G. M., & Quick, G. W. (2010). Fast versatile regeneration of Trifolium alexandrinum L. Plant Cell Tissue and Organ Culture, 100, 39–48.
Bhowal, M., Cherian, K. J., & Das, L. (2011). Direct organogenesis in fodder crop Trifolium alexandruim L. Journal of Environment Research and Development, 5, 892–897.
Tanaka, N., Fujikawa, Y., Aly, M. A. M., Saneoka, H., Fujita, K., & Yamashita, I. (2001). Proliferation and rol gene expression in hairy root lines of Egyptian clover. Plant Cell Tissue and Organ Culture, 66, 175–182.
Moriuchi, H., Fujikawa, Y., Mohammed, A. L. Y., Saneoka, H., Fujita, K., Yamashitai, I., & Tanaka, N. (2004). Hairy root-mediated transgenic-plant regeneration in Egyptian clover (Trifolium alexandrinum L.). Plant Biotechenology, 21, 165–168.
Abogadallah, G. M., Nada, R. M., Malinowski, R., & Quick, P. (2011). Overexpression of HARDY, an AP2/ERF gene from Arabidopsis, improves drought and salt tolerance by reducing transpiration and sodium uptake in transgenic Trifolium alexandrinum L. Planta, 233, 1265–1276.
Moghaieb, R., Abdelazim, A., Youssef, S., Ibrahim, S., & Hussein, B. (2014). Regeneration and transformation efficiencies among five Egyptian clover cultivars (Trifolium alexandrinum). International Journal of Advanced Research, 2, 227–233.
Anjum, N., Joyia, F., Mustafa, G., Ishtiaq, R., Ali, M., Amjad, M. S., & Khan, M. S. (2018). Direct in vitro regeneration and transient Gus assay: Towards stable genetic transformation in Trifolium alexandrinum L. Yyu J. Agricul. Sci., 28, 315–320.
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory Press.
Jyothishwaran, G., Kotresha, D., Selvaraj, T., Srideshikan, S. M., Rajvanshi, P. K., & Jayabaskaran, C. (2007). A modified freeze–thaw method for efficient transformation of Agrobacterium tumefaciens. Current Science, 93, 770–772.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum, 15, 473–497.
Gamborg, O. L., Miller, R. A., & Ojima, K. (1968). Nutrient requirements of suspension cultures of soybean root cells. Experimental Cell Research, 50, 151–158.
Rogers, S. O., & Bendich, A. J. (1989). Extraction of DNA from plant tissues. In S. Gelvin & R. A. Schilperoort (Eds.), Plant molecular biology manual (pp. 73–83). Kluwer academic publishers.
Bradford, M. M. (1976). A rapid and sensitive method for the quantisation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Sivanandhan, G., Kapil Dev, G., Theboral, J., Selvaraj, N., Ganapathi, A., & Manickavasagam, M. (2015). Sonication, vacuum infiltration and thiol compounds enhance the agrobacterium-mediated transformation frequency of Withania somnifera (L.) Dunal. PLoS One, 10, e0124693. https://doi.org/10.1371/journal.pone.0124693
Ding, Y.-L., Aldao-Humble, G., Ludlow, E., Drayton, M., Lin, Y.-H., Nagel, J., Dupal, M., Zhao, G., Pallaghy, C., Kalla, R., et al. (2003). Efficient plant regeneration and Agrobacterium-mediated transformation in Medicago and Trifolium species. Plant Science, 165, 1419–1427.
Richardson, K. A., Maher, D. A., Jones, C. S., et al. (2013). Genetic transformation of western clover (Trifolium occidentale D. E. Coombe.) as a model for functional genomics and transgene introgression in clonal pasture legume species. Plant Methods, 9, 25. https://doi.org/10.1186/1746-4811-9-25
Rojo, F. P., Seth, S., Erskine, W., & Kaur, P. (2021). An improved protocol for Agrobacterium-mediated transformation in subterranean clover (Trifolium subterraneum L). International Journal of Molecular Sciences, 22, 4181. https://doi.org/10.3390/ijms22084181
Zhou, B., Zhang, Y., Wang, X., Dong, J., Wang, B., Han, C., Yu, J., & Li, D. (2010). Oral administration of plant-based rotavirus VP6 induces antigen-specific IgAs, IgGs and passive protection in mice. Vaccine, 28, 6021–6027.
Esteves, P. J., Abrantes, J., Baldauf, H. M., et al. (2018). The wide utility of rabbits as models of human diseases. Experimental & Molecular Medicine, 50, 1–10. https://doi.org/10.1038/s12276-018-0094-1
Heinimäki, S., Tamminen, K., Hytönen, V. P., Malm, M., & Blazevic, V. (2020). Rotavirus inner capsid VP6 acts as an adjuvant in formulations with particulate antigens only. Vaccines, 8(3), 365. https://doi.org/10.3390/vaccines8030365
Acknowledgements
PKJ and PM express their gratitude to the University Grants Commission, New Delhi, for the award of BSR Faculty Fellowship (18-1/2011(BSR)) and Senior Research Fellowship, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they do not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Malik, P., Prajapati, M., Chaudhary, D. et al. Production of Bovine Rotavirus VP6 Subunit Vaccine in a Transgenic Fodder Crop, Egyptian Clover (Berseem, Trifolium alexandrinum) that Elicits Immune Responses in Rabbit. Mol Biotechnol 65, 1432–1443 (2023). https://doi.org/10.1007/s12033-022-00648-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-022-00648-0