Abstract
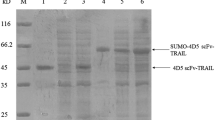
Autoinduction is a simple approach for heterologous protein expression that helps to achieve the high-level production of recombinant proteins in soluble form. In this work, we investigated if the application of an autoinduction strategy could help to optimize the production of bifunctional protein SRH-DR5-B, the DR5-specific TRAIL variant DR5-B fused to a VEGFR2-specific peptide SRHTKQRHTALH for dual antitumor and antiangiogenic activity. The protein was expressed in Escherichia coli SHuffle B T7, BL21(DE3), and BL21(DE3)pLysS strains. By IPTG induction, the highest expression level was in SHuffle B T7, while by autoinduction, the similar expression level was achieved in BL21(DE3)pLysS. However, in SHuffle B T7, only 45% of IPTG-induced SRH-DR5-B was expressed in soluble form, in contrast to 75% autoinduced in BL21(DE3)pLysS. The yield of purified SRH-DR5-B protein expressed by autoinduction in BL21(DE3)pLysS was 28 ± 4.5 mg per 200 ml of cell culture, which was 1.4 times higher than the yield from IPTG-induced SHuffle B T7. Regardless of the production method, SRH-DR5-B was equally cytotoxic to BxPC-3 human tumor cells expressing DR5 and VEGFR2 receptors. Thus, the production of SRH-DR5-B by autoinduction in the E. coli BL21(DE3)pLysS strain is an efficient, technologically simple, and economical technique that allows to obtain a large amount of active protein from the cytoplasmic cell fraction. Our work demonstrates that the strategy of induction of protein expression is no less important than the strain selection.




Similar content being viewed by others
References
Dianat-Moghadam, H., Heidarifard, M., Mahari, A., Shahgolzari, M., Keshavarz, M., Nouri, M., & Amoozgar, Z. (2020). TRAIL in oncology: From recombinant TRAIL to nano- and self-targeted TRAIL-based therapies. Pharmacological Research, 155, 104716. https://doi.org/10.1016/j.phrs.2020.104716
Krishna Moorthy, N., Seifert, O., Eisler, S., Weirich, S., Kontermann, R. E., Rehm, M., & Fullstone, G. (2021). Low-level endothelial TRAIL-receptor expression obstructs the CNS-delivery of angiopep-2 functionalised TRAIL-receptor agonists for the treatment of glioblastoma. Molecules, 26(24), 7582. https://doi.org/10.3390/molecules26247582
Bremer, E., Samplonius, D. F., van Genne, L., Dijkstra, M. H., Kroesen, B. J., de Leij, L. F. M. H., & Helfrich, W. (2005). Simultaneous inhibition of epidermal growth factor receptor (EGFR) signaling and enhanced activation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor-mediated apoptosis induction by an scFv:STRAIL fusion protein with specificity for human EGFR. Journal of Biological Chemistry, 280(11), 10025–10033. https://doi.org/10.1074/jbc.M413673200
de Bruyn, M., Rybczynska, A. A., Wei, Y., Schwenkert, M., Fey, G. H., Dierckx, R. A., & Bremer, E. (2010). Melanoma-associated chondroitin sulfate proteoglycan (MCSP)-targeted delivery of soluble TRAIL potently inhibits melanoma outgrowth in vitro and in vivo. Molecular Cancer, 9(1), 301. https://doi.org/10.1186/1476-4598-9-301
Hendriks, D., He, Y., Koopmans, I., Wiersma, V. R., van Ginkel, R. J., Samplonius, D. F., & Bremer, E. (2016). Programmed death ligand 1 (PD-L1)-targeted TRAIL combines PD-L1-mediated checkpoint inhibition with TRAIL-mediated apoptosis induction. OncoImmunology, 5(8), e1202390. https://doi.org/10.1080/2162402X.2016.1202390
Rozanov, D., Spellman, P., Savinov, A., & Strongin, A. Y. (2015). A humanized leucine zipper-TRAIL hybrid induces apoptosis of tumors both in vitro and in vivo. PLoS ONE, 10(4), e0122980. https://doi.org/10.1371/journal.pone.0122980
Wang, X., Qiao, X., Shang, Y., Zhang, S., Li, Y., He, H., & Chen, S. (2017). RGD and NGR modified TRAIL protein exhibited potent anti-metastasis effects on TRAIL-insensitive cancer cells in vitro and in vivo. Amino Acids, 49(5), 931–941. https://doi.org/10.1007/s00726-017-2395-4
Tripathi, N. K., & Shrivastava, A. (2019). Recent developments in bioprocessing of recombinant proteins: Expression hosts and process development. Frontiers in Bioengineering and Biotechnology, 7, 420. https://doi.org/10.3389/fbioe.2019.00420
Vincentelli, R., & Romier, C. (2013). Expression in Escherichia coli: Becoming faster and more complex. Current Opinion in Structural Biology, 23(3), 326–334. https://doi.org/10.1016/j.sbi.2013.01.006
Jia, B., & Jeon, C. O. (2016). High-throughput recombinant protein expression in Escherichia coli : Current status and future perspectives. Open Biology, 6(8), 160196. https://doi.org/10.1098/rsob.160196
Gasparian, M. E., Ostapchenko, V. G., Yagolovich, A. V., Tsygannik, I. N., Chernyak, B. V., Dolgikh, D. A., & Kirpichnikov, M. P. (2007). Overexpression and refolding of thioredoxin/TRAIL fusion from inclusion bodies and further purification of TRAIL after cleavage by enteropeptidase. Biotechnology Letters, 29(10), 1567–1573. https://doi.org/10.1007/s10529-007-9446-y
Li, P., Gu, Q., & Wu, X. (2016). Fed-batch production of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in soluble form in Escherichia coli and its purification and characterization. Protein Expression and Purification, 126, 115–121. https://doi.org/10.1016/j.pep.2016.06.007
Zhang, M., Wang, Z., Chi, L., Sun, J., & Shen, Y. (2018). Enhanced production of soluble tumor necrosis factor-related apoptosis-inducing ligand in Escherichia coli using a novel self-cleavable tag system Fh8-ΔI-CM. Protein Expression and Purification, 148, 16–23. https://doi.org/10.1016/j.pep.2018.03.005
Li, R., Yang, H., Jia, D., Nie, Q., Cai, H., Fan, Q., & Lu, X. (2016). Fusion to an albumin-binding domain with a high affinity for albumin extends the circulatory half-life and enhances the in vivo antitumor effects of human TRAIL. Journal of Controlled Release, 228, 96–106. https://doi.org/10.1016/j.jconrel.2016.03.004
Tao, Z., Yang, H., Shi, Q., Fan, Q., Wan, L., & Lu, X. (2017). Targeted delivery to tumor-associated pericytes via an affibody with high affinity for PDGFRβ enhances the in vivo antitumor effects of human TRAIL. Theranostics, 7(8), 2261–2276. https://doi.org/10.7150/thno.19091
Yang, H., Feng, Y., Cai, H., Jia, D., Li, H., Tao, Z., & Lu, X. (2018). Endogenous IgG-based affinity-controlled release of TRAIL exerts superior antitumor effects. Theranostics, 8(9), 2459–2476. https://doi.org/10.7150/thno.23880
Li, Z., She, T., Yang, H., Su, T., Shi, Q., Tao, Z., & Lu, X. (2022). A novel tumor-homing TRAIL variant eradicates tumor xenografts of refractory colorectal cancer cells in combination with tumor cell-targeted photodynamic therapy. Drug Delivery, 29(1), 1698–1711. https://doi.org/10.1080/10717544.2022.2079766
Wang, Y., Lei, Q., Yan, Z., Shen, C., & Wang, N. (2018). TGF3L fusion enhances the antitumor activity of TRAIL by promoting assembly into polymers. Biochemical Pharmacology, 155, 510–523. https://doi.org/10.1016/j.bcp.2018.07.035
Wang, Y., Lei, Q., Shen, C., & Wang, N. (2021). NCTR25 fusion facilitates the formation of TRAIL polymers that selectively activate TRAIL receptors with higher potency and efficacy than TRAIL. Cancer Chemotherapy and Pharmacology, 88(2), 289–306. https://doi.org/10.1007/s00280-021-04283-5
Madhumathi, J., Sridevi, S., & Verma, R. S. (2016). Novel TNF-related apoptotic-inducing ligand-based immunotoxin for therapeutic targeting of CD25 positive leukemia. Targeted Oncology, 11(4), 535–547. https://doi.org/10.1007/s11523-016-0424-y
Brin, E., Wu, K., Dagostino, E., Meng-Chiang Kuo, M., He, Y., Shia, W.-J., & Thomson, J. (2018). TRAIL stabilization and cancer cell sensitization to its pro-apoptotic activity achieved through genetic fusion with arginine deiminase. Oncotarget, 9(97), 36914–36928. https://doi.org/10.18632/oncotarget.26398
Gaglione, R., Pane, K., Dell’Olmo, E., Cafaro, V., Pizzo, E., Olivieri, G., & Arciello, A. (2019). Cost-effective production of recombinant peptides in Escherichia coli. New Biotechnology, 51, 39–48. https://doi.org/10.1016/j.nbt.2019.02.004
Gasparian, M. E., Chernyak, B. V., Dolgikh, D. A., Yagolovich, A. V., Popova, E. N., Sycheva, A. M., & Kirpichnikov, M. P. (2009). Generation of new TRAIL mutants DR5-A and DR5-B with improved selectivity to death receptor 5. Apoptosis, 14(6), 778–787. https://doi.org/10.1007/s10495-009-0349-3
Yagolovich, A. V., Artykov, A. A., Isakova, A. A., Vorontsova, Y. V., Dolgikh, D. A., Kirpichnikov, M. P., & Gasparian, M. E. (2022). Optimized heterologous expression and efficient purification of a new TRAIL-based antitumor fusion protein SRH–DR5-B with dual VEGFR2 and DR5 receptor specificity. International Journal of Molecular Sciences, 23(11), 5860. https://doi.org/10.3390/ijms23115860
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning: A laboratory manual (2nd ed.). Cold Spring Harbor laboratory press.
Yagolovich, A. V., Artykov, A. A., Dolgikh, D. A., Kirpichnikov, M. P., & Gasparian, M. E. (2019). A new efficient method for production of recombinant antitumor cytokine TRAIL and its receptor-selective variant DR5-B. Biochemistry (Moscow), 84(6), 627–636. https://doi.org/10.1134/S0006297919060051
Studier, F. W. (2005). Protein production by auto-induction in high-density shaking cultures. Protein Expression and Purification, 41(1), 207–234. https://doi.org/10.1016/j.pep.2005.01.016
Lobstein, J., Emrich, C. A., Jeans, C., Faulkner, M., Riggs, P., & Berkmen, M. (2012). SHuffle, a novel Escherichia coli protein expression strain capable of correctly folding disulfide bonded proteins in its cytoplasm. Microbial Cell Factories, 11(1), 753. https://doi.org/10.1186/1475-2859-11-56
Ueyama, H., Horibe, T., Nakajima, O., Ohara, K., Kohno, M., & Kawakami, K. (2011). Semaphorin 3A lytic hybrid peptide binding to neuropilin-1 as a novel anti-cancer agent in pancreatic cancer. Biochemical and Biophysical Research Communications, 414(1), 60–66. https://doi.org/10.1016/j.bbrc.2011.09.021
Mohr, A., Yu, R., & Zwacka, R. M. (2015). TRAIL-receptor preferences in pancreatic cancer cells revisited: Both TRAIL-R1 and TRAIL-R2 have a licence to kill. BMC Cancer, 15(1), 494. https://doi.org/10.1186/s12885-015-1508-2
Gopal, G. J., & Kumar, A. (2013). Strategies for the production of recombinant protein in Escherichia coli. The Protein Journal, 32(6), 419–425. https://doi.org/10.1007/s10930-013-9502-5
Structural Genomics Consortium, Architecture et Fonction des Macromolécules Biologiques, Berkeley Structural Genomics Center, China Structural Genomics Consortium, Integrated Center for Structure and Function Innovation, Israel Structural Proteomics Center, SPINE2-Complexes. (2008). Protein production and purification. Nature Methods, 5(2), 135–146. https://doi.org/10.1038/nmeth.f.202
Studier, F. W., & Moffatt, B. A. (1986). Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. Journal of Molecular Biology, 189(1), 113–130. https://doi.org/10.1016/0022-2836(86)90385-2
Grodberg, J., & Dunn, J. J. (1988). ompT encodes the Escherichia coli outer membrane protease that cleaves T7 RNA polymerase during purification. Journal of Bacteriology, 170(3), 1245–1253. https://doi.org/10.1128/jb.170.3.1245-1253.1988
Gottesman, S. (1996). Proteases and their targets in Escherichia coli. Annual Review of Genetics, 30(1), 465–506. https://doi.org/10.1146/annurev.genet.30.1.465
Stano, N. M., & Patel, S. S. (2004). T7 lysozyme represses T7 RNA polymerase transcription by destabilizing the open complex during initiation. Journal of Biological Chemistry, 279(16), 16136–16143. https://doi.org/10.1074/jbc.M400139200
Hatahet, F., Boyd, D., & Beckwith, J. (2014). Disulfide bond formation in prokaryotes: History, diversity and design. Biochimica et Biophysica Acta (BBA)—Proteins and Proteomics, 1844(8), 1402–1414. https://doi.org/10.1016/j.bbapap.2014.02.014
Ren, G., Ke, N., & Berkmen, M. (2016). Use of the SHuffle strains in production of proteins. Current Protocols in Protein Science. https://doi.org/10.1002/cpps.11
Nozach, H., Fruchart-Gaillard, C., Fenaille, F., Beau, F., Ramos, O. H. P., Douzi, B., & Dive, V. (2013). High throughput screening identifies disulfide isomerase DsbC as a very efficient partner for recombinant expression of small disulfide-rich proteins in E. coli. Microbial Cell Factories, 12(1), 37. https://doi.org/10.1186/1475-2859-12-37
Nikolova, G., Georgieva, Y., Atanasova, A., Radulova, G., Kapogianni, A., & Tsacheva, I. (2021). Autoinduction as means for optimization of the heterologous expression of recombinant single-chain Fv (scFv) antibodies. Molecular Biotechnology, 63(11), 1049–1056. https://doi.org/10.1007/s12033-021-00363-2
Ding, N., Yang, C., Sun, S., Han, L., Ruan, Y., Guo, L., & Zhang, J. (2017). Increased glycosylation efficiency of recombinant proteins in Escherichia coli by auto-induction. Biochemical and Biophysical Research Communications, 485(1), 138–143. https://doi.org/10.1016/j.bbrc.2017.02.037
Fathi-Roudsari, M., Maghsoudi, N., Maghsoudi, A., Niazi, S., & Soleiman, M. (2018). Auto-induction for high level production of biologically active reteplase in Escherichia coli. Protein Expression and Purification, 151, 18–22. https://doi.org/10.1016/j.pep.2018.05.008
Wu, H., Chen, B., Jiang, H., Wu, L., Zhu, L.-Y., Meng, E., & Zhang, D.-Y. (2017). Heterologous expression and purification of neurotoxic Hainantoxin-III in E. coli. Preparative Biochemistry & Biotechnology, 47(2), 158–162. https://doi.org/10.1080/10826068.2016.1188313
Nair, R., Salvi, P., Banerjee, S., Raiker, V. A., Bandyopadhyay, S., Soorapaneni, S., & Padmanabhan, S. (2009). Yeast extract mediated autoinduction of lacUV5 promoter: An insight. New Biotechnology, 26(6), 282–288. https://doi.org/10.1016/j.nbt.2009.08.002
Shirano, Y., & Shibata, D. (1990). Low temperature cultivation of Escherichia coli carrying a rice lipoxygenase L-2 cDNA produces a soluble and active enzyme at a high level. FEBS Letters, 271(1–2), 128–130. https://doi.org/10.1016/0014-5793(90)80388-Y
Yang, X., & Zhang, Y. (2013). Effect of temperature and sorbitol in improving the solubility of carboxylesterases protein CpCE-1 from Cydia pomonella and biochemical characterization. Applied Microbiology and Biotechnology, 97(24), 10423–10433. https://doi.org/10.1007/s00253-013-5236-8
Gupta, S. K., & Shukla, P. (2016). Advanced technologies for improved expression of recombinant proteins in bacteria: Perspectives and applications. Critical Reviews in Biotechnology, 36(6), 1089–1098. https://doi.org/10.3109/07388551.2015.1084264
Lu, Z., Chen, W., Liu, R., Hu, X., & Ding, Y. (2010). A novel method for high-level production of psychrophilic TAB5 alkaline phosphatase. Protein Expression and Purification, 74(2), 217–222. https://doi.org/10.1016/j.pep.2010.06.010
Chen, X., Wu, J., Liu, H., He, Z., Gu, M., Wang, N., & Zhu, X. (2010). Approaches to efficient production of recombinant angiogenesis inhibitor rhVEGI-192 and characterization of its structure and antiangiogenic function: RhVEGI-192 and its antiangiogenic activity. Protein Science, 19(3), 449–457. https://doi.org/10.1002/pro.323
Yu, S., Wang, Y., Liu, Y., Mo, W., Song, H., & Yu, M. (2009). Expression and purification of APRIL by auto-induction. Protein Expression and Purification, 68(1), 49–53. https://doi.org/10.1016/j.pep.2009.06.009
Pulido, I. Y., Prieto, E., Pieffet, G. P., Méndez, L., & Jiménez-Junca, C. A. (2020). Functional heterologous expression of mature lipase lipA from Pseudomonas aeruginosa PSA01 in Escherichia coli SHuffle and BL21 (DE3): Effect of the expression host on thermal stability and solvent tolerance of the enzyme produced. International Journal of Molecular Sciences, 21(11), 3925. https://doi.org/10.3390/ijms21113925
Acknowledgements
The research was funded by Russian Science Foundation grant No. 21-14-00224, https://rscf.ru/project/21-14-00224/.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Isakova, A., Artykov, A., Vorontsova, Y. et al. Application of an Autoinduction Strategy to Optimize the Heterologous Production of an Antitumor Bispecific Fusion Protein Based on the TRAIL Receptor-Selective Mutant Variant in Escherichia coli. Mol Biotechnol 65, 581–589 (2023). https://doi.org/10.1007/s12033-022-00561-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-022-00561-6