Abstract
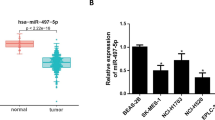
MiR-99a-5p participates in processes and pathogenesis of varying diseases. However, the molecular mechanism of miR-99a-5p in human cervical squamous cell carcinoma (CSCC) remains unclear. Here, we found that miR-99a-5p was lowly expressed in CSCC cells and negatively associated with overall survival. In addition, cellular experiments including CCK8, wound healing, Transwell and flow cytometry assays disclosed that transfection of miR-99a-5p mimic could suppress the cell activity, cell migratory, and invasive abilities, and promote cell apoptosis, thus inhibiting the tumor progression of CSCC cells. Luciferase reporter gene assay indicated that miR-99a-5p targeted 3’-UTR of CDC25A. Also, enforced CDC25A level rescued the impact of miR-99a-5p on CSCC progression. Silencing CDC25A could restrain the mRNA and protein levels of IL-6 in CSCC. CDC25A overexpression or IL-6 treatment could attenuate inhibiting impact of miR-99a-5p overexpression on epithelial–mesenchymal transition (EMT). These findings suggested that miR-99a-5p may play an anti-tumor role in tumor metastasis by targeting CDC25A/IL6 to hamper EMT process, which revealed a novel molecular mechanism in CSCC.





Similar content being viewed by others
Data Availability
The data used to support the findings of this study are included within the article. The data and materials in the current study are available from the corresponding author on reasonable request.
References
Cancer Genome Atlas Research, et al. (2017). Integrated genomic and molecular characterization of cervical cancer. Nature, 543, 378–384. https://doi.org/10.1038/nature21386
Sung, H., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71, 209–249. https://doi.org/10.3322/caac.21660
Waggoner, S. E. (2003). Cervical cancer. Lancet, 361, 2217–2225. https://doi.org/10.1016/S0140-6736(03)13778-6
Crosbie, E. J., Einstein, M. H., Franceschi, S., & Kitchener, H. C. (2013). Human papillomavirus and cervical cancer. Lancet, 382, 889–899. https://doi.org/10.1016/S0140-6736(13)60022-7
Marth, C., et al. (2017). Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 28, 72–83. https://doi.org/10.1093/annonc/mdx220
Zhu, H., et al. (2016). Molecular mechanisms of cisplatin resistance in cervical cancer. Drug Des Devel Ther, 10, 1885–1895. https://doi.org/10.2147/DDDT.S106412
Croce, C. M. (2009). Causes and consequences of microRNA dysregulation in cancer. Nature Reviews Genetics, 10, 704–714. https://doi.org/10.1038/nrg2634
Dillhoff, M., Liu, J., Frankel, W., Croce, C., & Bloomston, M. (2008). MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. Journal of Gastrointestinal Surgery, 12, 2171–2176. https://doi.org/10.1007/s11605-008-0584-x
Li, J., et al. (2012). MicroRNA-223 functions as an oncogene in human gastric cancer by targeting FBXW7/hCdc4. Journal of Cancer Research and Clinical Oncology, 138, 763–774. https://doi.org/10.1007/s00432-012-1154-x
Zhu, L., Tu, H., Liang, Y., & Tang, D. (2018). MiR-218 produces anti-tumor effects on cervical cancer cells in vitro. World J Surg Oncol, 16, 204. https://doi.org/10.1186/s12957-018-1506-3
Jiang, L., et al. (2018). MicroRNA-519d-3p Inhibits Proliferation and Promotes Apoptosis by Targeting HIF-2α in Cervical Cancer Under Hypoxic Conditions. Oncology research, 26, 1055–1062. https://doi.org/10.3727/096504018x15152056890500
Li, P., et al. (2018). miR-182 promotes cell proliferation of cervical cancer cells by targeting adenomatous polyposis coli (APC) gene. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi, 34, 148–153.
Galaktionov, K., & Beach, D. (1991). Specific activation of cdc25 tyrosine phosphatases by B-type cyclins: Evidence for multiple roles of mitotic cyclins. Cell, 67, 1181–1194. https://doi.org/10.1016/0092-8674(91)90294-9
Lavecchia, A., Di Giovanni, C., & Novellino, E. (2010). Inhibitors of Cdc25 phosphatases as anticancer agents: A patent review. Expert Opinion on Therapeutic Patents, 20, 405–425. https://doi.org/10.1517/13543771003623232
Qin, H., & Liu, W. (2019). MicroRNA-99a-5p suppresses breast cancer progression and cell-cycle pathway through downregulating CDC25A. Journal of Cellular Physiology, 234, 3526–3537. https://doi.org/10.1002/jcp.26906
Al-Matouq, J., et al. (2017). Accumulation of cytoplasmic CDC25A in cutaneous squamous cell carcinoma leads to a dependency on CDC25A for cancer cell survival and tumor growth. Cancer Letters, 410, 41–49. https://doi.org/10.1016/j.canlet.2017.09.023
Bertoli, S., et al. (2015). CDC25A governs proliferation and differentiation of FLT3-ITD acute myeloid leukemia. Oncotarget, 6, 38061–38078. https://doi.org/10.18632/oncotarget.5706
Ataie-Kachoie, P., Pourgholami, M. H., Richardson, D. R., & Morris, D. L. (2014). Gene of the month: Interleukin 6 (IL-6). Journal of Clinical Pathology, 67, 932–937. https://doi.org/10.1136/jclinpath-2014-202493
Miao, J. W., Liu, L. J., & Huang, J. (2014). Interleukin-6-induced epithelial-mesenchymal transition through signal transducer and activator of transcription 3 in human cervical carcinoma. International Journal of Oncology, 45, 165–176. https://doi.org/10.3892/ijo.2014.2422
Chen, S., et al. (2020). Silencing CDC25A inhibits the proliferation of liver cancer cells by downregulating IL6 in vitro and in vivo. International Journal of Molecular Medicine, 45, 743–752. https://doi.org/10.3892/ijmm.2020.4461
Robova, H., Rob, L., Halaska, M. J., Pluta, M., & Skapa, P. (2015). Review of neoadjuvant chemotherapy and trachelectomy: Which cervical cancer patients would be suitable for neoadjuvant chemotherapy followed by fertility-sparing surgery? Current Oncology Reports, 17, 446. https://doi.org/10.1007/s11912-015-0446-0
Kogo, R., et al. (2015). The microRNA-218~Survivin axis regulates migration, invasion, and lymph node metastasis in cervical cancer. Oncotarget, 6, 1090–1100. https://doi.org/10.18632/oncotarget.2836
Vandeperre, A., et al. (2015). Para-aortic lymph node metastases in locally advanced cervical cancer: Comparison between surgical staging and imaging. Gynecologic Oncology, 138, 299–303. https://doi.org/10.1016/j.ygyno.2015.05.021
Wong, H. A., et al. (2015). The cancer genome atlas analysis predicts MicroRNA for targeting cancer growth and vascularization in glioblastoma. Molecular Therapy, 23, 1234–1247. https://doi.org/10.1038/mt.2015.72
Tsai, T. F., et al. (2018). miR-99a-5p acts as tumor suppressor via targeting to mTOR and enhances RAD001-induced apoptosis in human urinary bladder urothelial carcinoma cells. Oncotargets and Therapy, 11, 239–252. https://doi.org/10.2147/OTT.S114276
Shi, Y., et al. (2017). MiR-99a-5p regulates proliferation, migration and invasion abilities of human oral carcinoma cells by targeting NOX4. Neoplasma, 64, 666–673. https://doi.org/10.4149/neo_2017_503
Chen, Y. T., et al. (2018). Biological role and clinical value of miR-99a-5p in head and neck squamous cell carcinoma (HNSCC): A bioinformatics-based study. FEBS Open Bio, 8, 1280–1298. https://doi.org/10.1002/2211-5463.12478
Liu, Y., Li, B., Yang, X., & Zhang, C. (2019). MiR-99a-5p inhibits bladder cancer cell proliferation by directly targeting mammalian target of rapamycin and predicts patient survival. Journal of Cellular Biochemistry, 120, 19330–19337. https://doi.org/10.1002/jcb.27318
Sun, X., & Yan, H. (2021). MicroRNA-99a-5p suppresses cell proliferation, migration, and invasion by targeting isoprenylcysteine carboxylmethyltransferase in oral squamous cell carcinoma. Journal of International Medical Research, 49, 300060520939031. https://doi.org/10.1177/0300060520939031
Kiyokawa, H., & Ray, D. (2008). In vivo roles of CDC25 phosphatases: Biological insight into the anti-cancer therapeutic targets. Anti-Cancer Agents in Medicinal Chemistry, 8, 832–836. https://doi.org/10.2174/187152008786847693
Dozier, C., et al. (2017). CyclinD-CDK4/6 complexes phosphorylate CDC25A and regulate its stability. Oncogene, 36, 3781–3788. https://doi.org/10.1038/onc.2016.506
Liu, W., et al. (2018). SIRT6 inhibits colorectal cancer stem cell proliferation by targeting CDC25A. Oncology Letters, 15, 5368–5374. https://doi.org/10.3892/ol.2018.7989
Biswas, S. C., Sanphui, P., Chatterjee, N., Kemeny, S., & Greene, L. A. (2017). Cdc25A phosphatase: A key cell cycle protein that regulates neuron death in disease and development. Cell Death & Disease, 8, e2692. https://doi.org/10.1038/cddis.2017.115
Jiang, T., & Cheng, H. (2021). miR-34a-5p blocks cervical cancer growth and migration by downregulating CDC25A. Journal of B.U.ON., 26, 1768–1774.
Fu, S., & Lin, J. (2018). Blocking Interleukin-6 and Interleukin-8 signaling inhibits cell viability, colony-forming activity, and cell migration in human triple-negative breast cancer and pancreatic cancer cells. Anticancer Research, 38, 6271–6279. https://doi.org/10.21873/anticanres.12983
Huang, Q., et al. (2018). 17beta-estradiol upregulates IL6 expression through the ERbeta pathway to promote lung adenocarcinoma progression. Journal of Experimental & Clinical Cancer Research, 37, 133. https://doi.org/10.1186/s13046-018-0804-5
Wei, L. H., et al. (2001). Interleukin-6 in cervical cancer: The relationship with vascular endothelial growth factor. Gynecologic Oncology, 82, 49–56. https://doi.org/10.1006/gyno.2001.6235
Acknowledgements
Not applicable.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no potential conflicts of interest.
Ethical Approval
Not applicable.
Consent for Publication
All authors consent to submit the manuscript for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gu, A., Bao, X. MiR-99a-5p Constrains Epithelial–Mesenchymal Transition of Cervical Squamous Cell Carcinoma Via Targeting CDC25A/IL6. Mol Biotechnol 64, 1234–1243 (2022). https://doi.org/10.1007/s12033-022-00496-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-022-00496-y