Abstract
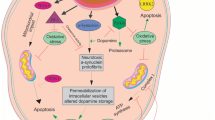
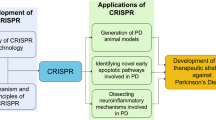
Genetic studies of familial forms of Parkinson’s disease (PD) have shown that the ZNF543 gene is a candidate gene that operates relevant to this disease. However, until now, there is no evidence for ZNF543 gene function in PD, and mechanisms resulting from its mutation have not been elucidated. Given the same genetic location of the ZNF543 gene with TRIM28 and their effects on PD pathogenesis, we surmised that ZNF543 might act as a transcription factor for TRIM28 gene expression. By knocking out the ZNF543 gene via the CRISPR/Cas9 editing platform, we assessed the functional effect of loss of expression of this gene on TRIM28 gene expression. Four sgRNAs with different PAM sequences were designed against two parts of the regulatory region of ZNF543 gene, and highly efficient disruption of ZNF543 expression in human neuroblastoma cell line was evaluated by polymerase chain reaction and T7 endonuclease assay. Moreover, evaluation of TRIM28 gene expression in ZNF543-knocked-out cells indicated a significant increase in TRIM28 gene expression, suggesting that ZNF543 probably regulates the expression of TRIM28. This approach offers a window into pinpointing the mechanism by which ZNF543 gene mutations mediate PD pathogenicity.





Similar content being viewed by others
Data Availability
The data sets generated and analyzed in the current study are available from the corresponding author on reasonable request.
References
Lau, De., Lonneke, M. L., & Breteler, M. M. B. (2006). Epidemiology of Parkinson’s disease. Lancet Neurology, 5, 525–535.
Klemann, C. J. H. M., Martens, G. J. M., Sharma, M., Martens, M. B., Isacson, O., Gasser, T., Visser, J. E., & Poelmans, G. (2017). Integrated molecular landscape of Parkinson’s disease. NPJ Parkinsons Diseases, 3, 1–7.
Bonifati, V. (2014). Genetics of Parkinson’s disease–state of the art, 2013. Parkinsonism & Related Disorders, 20, S23–S28.
Sasan, H., Hashemabadi, M., Amandadi, M., & Ravan, H. (2020). Alteration in the expression of Parkinson’s-related genes in rat hippocampus by exercise and morphine treatments. Russian Journal of Genetics (Translation of Genetika (Moscow, Russian Federation)), 56, 502–508.
Nalls, M. A., Blauwendraat, C., Vallerga, C. L., Heilbron, K., Bandres-Ciga, S., Chang, D., Tan, M., Kia, D. A., Noyce, A. J., & Xue, A. (2019). Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurology, 18, 1091–1102.
Jansen, I. E., Ye, H., Heetveld, S., Lechler, M. C., Michels, H., Seinstra, R. I., Lubbe, S. J., Drouet, V., Lesage, S., & Majounie, E. (2017). Discovery and functional prioritization of Parkinson’s disease candidate genes from large-scale whole exome sequencing. Genome Biology, 18, 1–26.
Simon-Sanchez, J., Schulte, C., Bras, J. M., Manu Sharma, J., Gibbs, R., Berg, D., Paisan-Ruiz, C., Lichtner, P., Scholz, S. W., & Hernandez, D. G. (2009). Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nature Genetics, 41, 1308.
Satake, W., Nakabayashi, Y., Mizuta, I., Hirota, Y., Ito, C., Kubo, M., Kawaguchi, T., Tsunoda, T., Watanabe, M., & Takeda, A. (2009). Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nature Genetics, 41, 1303.
Saad, M., Lesage, S., Saint-Pierre, A., Corvol, J.-C., Zelenika, D., Lambert, J.-C., Vidailhet, M., Mellick, G. D., Lohmann, E., & Durif, F. (2011). Genome-wide association study confirms BST1 and suggests a locus on 12q24 as the risk loci for Parkinson’s disease in the European population. Human Molecular Genetics, 20, 615–627.
Rousseaux, Maxime WC., de Haro, Maria, Lasagna-Reeves, Cristian A., De Maio, Antonia, Park, Jeehye, Jafar-Nejad, Paymaan, Al-Ramahi, Ismael, Sharma, Ajay, See, Lauren, & Nan, Lu. (2016). TRIM28 regulates the nuclear accumulation and toxicity of both alpha-synuclein and tau. Elife, 5, e19809.
Rousseaux, Maxime WC., Revelli, Jean-Pierre., Vázquez-Vélez, Gabriel E., Kim, Ji-Yoen., Craigen, Evelyn, Gonzales, Kristyn, Beckinghausen, Jaclyn, & Zoghbi, Huda Y. (2018). Depleting Trim28 in adult mice is well tolerated and reduces levels of α-synuclein and tau. Elife, 7, e36768.
Siddiqui, A., Chinta, S. J., Mallajosyula, J. K., Rajagopolan, S., Hanson, I., Rane, A., Melov, S., & Andersen, J. K. (2012). Selective binding of nuclear alpha-synuclein to the PGC1alpha promoter under conditions of oxidative stress may contribute to losses in mitochondrial function: Implications for Parkinson’s disease. Free Radical Biology & Medicine, 53, 993–1003.
Eschbach, J., Von Einem, B., Müller, K., Bayer, H., Scheffold, A., Morrison, B. E., Lenhard Rudolph, K., Thal, D. R., Witting, A., & Weydt, P. (2015). Mutual exacerbation of peroxisome proliferator-activated receptor γ coactivator 1α deregulation and α-synuclein oligomerization. Annals of Neurology, 77, 15–32.
Ecco, G., Imbeault, M., & Trono, D. (2017). KRAB zinc finger proteins. Development, 144, 2719–2729.
Joung, J. K., & Sander, J. D. (2013). TALENs: A widely applicable technology for targeted genome editing. Nature Reviews Molecular Cell Biology, 14, 49–55.
Gaj, T., Gersbach, C. A., & Barbas III, C. F. (2013). ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends in Biotechnology, 31, 397–405.
Mohammadinejad, R., Sassan, H., Pardakhty, A., Hashemabadi, M., Ashrafizadeh, M., Dehshahri, A., & Mandegary, A. (2020). ZEB1 and ZEB2 gene editing mediated by CRISPR/Cas9 in A549 cell line. Bratislavske Lekarske Listy, 121, 31–36.
Mohammadinejad, R., Biagioni, A., Arunkumar, G., Shapiro, R., Chang, K.-C., Sedeeq, M., Taiyab, A., Hashemabadi, M., Pardakhty, A., & Mandegary, A. (2020). EMT signaling: Potential contribution of CRISPR/Cas gene editing. Cellular and Molecular Life Sciences, 77, 2701–2722.
Samare Gholami, A., Sasan, H. A., Hashemabadi, M., & Ravan, H. (2019). Design and Construction of Recombinant CRISPR Vector Harboring LRRK2 Gene for Parkinson’s Disease. Cell and Tissue Research, 10, 214–225.
Riordan, S. M., Heruth, D. P., Zhang, L. Q., & Ye, S. Q. (2015). Application of CRISPR/Cas9 for biomedical discoveries. Cell & Bioscience, 5, 1–11.
Jiang, F., & Doudna, J. A. (2017). CRISPR–Cas9 structures and mechanisms. Annual Review of Biophysics, 46, 505–529.
Ran, F. A., Hsu, P. D., Wright, J., Agarwala, V., Scott, D. A., & Zhang, F. (2013). Genome engineering using the CRISPR-Cas9 system. Nature Protocols, 8, 2281–2308.
Rousseaux, M. W. C., de Haro, M., Lasagna-Reeves, C. A., De Maio, A., Jafar-Nejad, P., Park, J., Al-Ramahi, I., Kayed, R., Botas, H., & Zoghbi, H. Y. (2015). TRIM28 regulates the stability and toxicity of alpha-synuclein and tau through a common mechanism. Journal of the Neurological Sciences, 357, e285–e286.
Abak, A., Shoorei, H., Taheri, M., et al. (2021). ‘In vivo engineering of chromosome 19 q-arm by employing the CRISPR/AsCpf1 and ddAsCpf1 systems in human malignant gliomas (hypothesis). Journal of Molecular Neuroscience, 71, 1648–1663. https://doi.org/10.1007/s12031-021-01855-1
Helmschrodt, C., Höbel, S., Schöniger, S., Bauer, A., Bonicelli, J., Gringmuth, M., Fietz, S. A., Aigner, A., Richter, A., & Richter, F. (2017). Polyethylenimine nanoparticle-mediated siRNA delivery to reduce α-Synuclein expression in a model of Parkinson’s disease. Molecular Therapy-Nucleic Acids., 15(9), 57–68.
McCormack, A. L., Mak, S. K., Henderson, J. M., Bumcrot, D., Farrer, M. J., & Di Monte, D. A. (2010). α-synuclein suppression by targeted small interfering RNA in the primate substantia nigra. PloS one., 5(8), e12122.
Li, M., Xu, X., Chang, C. W., & Liu, Y. (2020). TRIM28 functions as the SUMO E3 ligase for PCNA in prevention of transcription induced DNA breaks. Proceedings of the National Academy of Sciences., 117(38), 23588–23596.
Gehrmann, U., Burbage, M., Zueva, E., Goudot, C., Esnault, C., Ye, M., Carpier, J. M., Burgdorf, N., Hoyler, T., Suarez, G., & Joannas, L. (2019). Critical role for TRIM28 and HP1β/γ in the epigenetic control of T cell metabolic reprograming and effector differentiation. Proceedings of the National Academy of Sciences., 116(51), 25839–25849.
Acknowledgements
The authors express appreciation to the Shahid Bahonar University of Kerman for its support of this investigation.
Funding
Not Applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. The design and writing of the manuscript were performed by MH and MA. Data collection was performed by MH, HS, and KE, and data analysis was performed by MH, SE, and HR.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All experiments and procedures were approved by the Animal Research Ethics Committee of the Kerman Neuroscience Research Center, Kerman, Iran (EC/KNRC/86–31).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hashemabadi, M., Sasan, H., Amandadi, M. et al. CRISPR/Cas9-Mediated Disruption of ZNF543 Gene: An Approach Toward Discovering Its Relation to TRIM28 Gene in Parkinson’s Disease. Mol Biotechnol 65, 243–251 (2023). https://doi.org/10.1007/s12033-022-00494-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-022-00494-0