Abstract
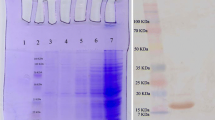
Immunotoxins have represented a great potency in targeted therapeutics to encounter tumors. They consist of a protein toxin conjugated to a targeting moiety, which recognizes a specific antigen on surface of cancer cells and accordingly induces cell death by toxin segment. The targeting part could be a nanobody, which is a group of antibodies composed of an only functional single variable heavy chain (VHH).Therefore, this study was done to produce an immunotoxin (VGRNb-DT) by chemical conjugation of a truncated diphtheria toxin moiety to an anti-vascular endothelial growth factor receptor 2(VEGFR-2) nanobody, and to identify effectiveness of immunotoxin in recognizing the VEGFR-2- positive cancer cells and inhibiting cell growth and survival. Diphtheria toxin was expressed and purified by nickel affinity chromatography, and accordingly, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis confirmed its expression. Function of heterobifunctional crosslinkers, Sulfo-SMCC (sulfosuccinimidyl-4-(N-maleimidomethyl) cyclohexane-1-carboxylate), and SATP (N-succinimidyl-S- acetylthiopropionate) for bioconjugation purposes was acknowledged by cation exchange high-performance liquid chromatography (HPLC). Cytotoxicity of immunotoxin was evaluated on the VEGFR-2 positive PC-3 cell line by MTT assay. Overexpression of VEGFR-2 in the PC-3 cell line allowed immunotoxin to recognize them by anti-VEGFR-2 nanobodies. The concentrations above 5 μg/ml represented a significant decrease in cell survival rate in PC-3 cells compared to HEK293 cells (VEGFR-2 negative cells) as controls.VGRNb-DT demonstrated a successful bioconjugation; furthermore, variable concentrations were correlated with cell death in prostate cancer PC-3 cells.





Similar content being viewed by others
Data Availability
The data used to support the findings of this study are included within the article.
References
Rawla, P. (2019). Epidemiology of prostate cancer. World journal of oncology, 10(2), 63.
Shafiee, F., Aucoin, M. G., & Jahanian Najafabadi, A. (2019). Targeted diphtheria toxin based therapy: A review article. Frontiers in microbiology. https://doi.org/10.3389/fmicb.2019.02340
Mei, X., et al. (2019). Immunotoxins: Targeted toxin delivery for cancer therapy. Pharmaceutical Fronts, 1(01), e33–e45.
Wenzel, E. V., et al. (2020). Human antibodies neutralizing diphtheria toxin in vitro and in vivo. Scientific reports, 10(1), 1–21.
Amoozadeh, S., et al. (2018). Determining induction conditions for expression of truncated diphtheria toxin and pseudomonas exotoxin A in E. coli BL21. Novelty in Biomedicine, 6(3), 131–137.
Bannas, P., Hambach, J., & Koch-Nolte, F. (2017). Nanobodies and nanobody-based human heavy chain antibodies as antitumor therapeutics. Frontiers in immunology, 8, 1603.
Sanaei, M., Setayesh, N., Sepehrizadeh, Z., Mahdavi, M., & Yazdi, M. H. (2020). Nanobodies in human infections: Prevention, detection, and treatment. Immunological Investigations, 49(8), 875–896. https://doi.org/10.1080/08820139.2019.1688828
Hu, Y., Liu, C., & Muyldermans, S. (2017). Nanobody-based delivery systems for diagnosis and targeted tumor therapy. Frontiers in immunology, 8, 1442.
Behdani, M., et al. (2012). Generation and characterization of a functional Nanobody against the vascular endothelial growth factor receptor-2; angiogenesis cell receptor. Molecular immunology, 50(1–2), 35–41.
Stadler, W. M., et al. (2004). A randomized Phase II trial of the antiangiogenic agent SU5416 in hormone-refractory prostate cancer. Clinical cancer research, 10(10), 3365–3370.
Reddy, V. G., et al. (2019). Pyrazolo-benzothiazole hybrids: Synthesis, anticancer properties and evaluation of antiangiogenic activity using in vitro VEGFR-2 kinase and in vivo transgenic zebrafish model. European journal of medicinal chemistry, 182, 111609.
Nordby, Y., et al. (2015). Stromal expression of VEGF-A and VEGFR-2 in prostate tissue is associated with biochemical and clinical recurrence after radical prostatectomy. The Prostate, 75(15), 1682–1693.
Guo, S., et al. (2010). Vascular endothelial growth factor receptor-2 in breast cancer. Biochimica et Biophysica Acta, 1806(1), 108–121.
Dev, I., et al. (2004). Antitumour efficacy of VEGFR2 tyrosine kinase inhibitor correlates with expression of VEGF and its receptor VEGFR2 in tumour models. British journal of cancer, 91(7), 1391–1398.
Amoozadeh, S., et al. (2019). Preparation of diphtheria and pseudomonas exotoxin a immunotoxins and evaluation of their cytotoxicity effect on SK-BR-3, BT-474, and MDA-MB-231 breast cancer cell lines. Cancer investigation, 37(10), 546–557.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227(5259), 680–685.
Hermanson, G. T. (2013). Bioconjugate techniques. Academic press.
Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of immunological methods, 65(1–2), 55–63.
Melincovici, C. S., et al. (2018). Vascular endothelial growth factor (VEGF)-key factor in normal and pathological angiogenesis. Romanian Journal of Morphology and Embryology, 59(2), 455–467.
Hegde, P.S., J.J. Wallin, and C. Mancao. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. in Seminars in cancer biology. 2018. Elsevier
Zhang, Y., et al. (2015). A novel bispecific immunotoxin delivered by human bone marrow-derived mesenchymal stem cells to target blood vessels and vasculogenic mimicry of malignant gliomas. Drug design, development and therapy, 9, 2947.
Langari, J., et al. (2017). In vitro evaluation of Vegf-Pseudomonas exotoxin: A conjugated on tumor cells. Advanced biomedical research, 6, 144.
Ran, S., et al. (2003). Evaluation of novel antimouse VEGFR2 antibodies as potential antiangiogenic or vascular targeting agents for tumor therapy. Neoplasia, 5(4), 297–307.
Frezzetti, D., et al. (2017). VEGF as a potential target in lung cancer. Expert Opinion on Therapeutic Targets, 21(10), 959–966.
Sarkar, C., et al. (2020). Angiogenesis inhibition in prostate cancer: An update. Cancers, 12(9), 2382.
Chen, T. T., et al. (2015). MET suppresses epithelial VEGFR2 via intracrine VEGF-induced endoplasmic reticulum-associated degradation. eBioMedicine, 2(5), 406–420.
Cha, H.-R., Lee, J. H., & Ponnazhagan, S. (2020). Revisiting immunotherapy: A focus on prostate cancer. Cancer research, 80(8), 1615–1623.
Deng, C., et al. (2017). Novel recombinant immunotoxin of EGFR specific nanobody fused with cucurmosin, construction and antitumor efficiency in vitro. Oncotarget, 8(24), 38568.
Cherubin, P., Quiñones, B., & Teter, K. (2018). Cellular recovery from exposure to sub-optimal concentrations of AB toxins that inhibit protein synthesis. Scientific reports, 8(1), 1–10.
Liu, Y., et al. (2016). VEGFR2 inhibition by RNA interference affects cell proliferation, migration, invasion, and response to radiation in Calu-1 cells. Clinical and Translational Oncology, 18(2), 212–219.
Xiang, Q., et al. (2014). Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and MET. Clinical Cancer Research, 20(11), 2959–2970.
Wüstemann, T., et al. (2019). Targeting prostate cancer: Prostate-specific membrane antigen based diagnosis and therapy. Medicinal research reviews, 39(1), 40–69.
Tai, S., et al. (2011). PC3 is a cell line characteristic of prostatic small cell carcinoma. The Prostate, 71(15), 1668–1679.
Zhang, F., et al. (2013). An anti-PSMA bivalent immunotoxin exhibits specificity and efficacy for prostate cancer imaging and therapy. Advanced healthcare materials, 2(5), 736–744.
Funding
This work was supported by the Iran University of Medical sciences, under grant number 33778.
Author information
Authors and Affiliations
Contributions
PT and MF: were responsible for the conception and design of the research. Methodology was done by all authors. Material preparation was performed by PT, MF, and MB. SS: performed the experiments and collected data. PT and MF: interpreted the results. SS: drafted manuscript and all the authors approved the draft of the submitted manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable since there are no human participants or animals.
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Consent for Publication
All the authors consent for the publication of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shajari, S., Farajollahi, M.M., Behdani, M. et al. Production and Conjugation of Truncated Recombinant Diphtheria Toxin to VEGFR-2 Specific Nanobody and Evaluation of its Cytotoxic Effect on PC-3 Cell Line. Mol Biotechnol 64, 1218–1226 (2022). https://doi.org/10.1007/s12033-022-00485-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-022-00485-1