Abstract
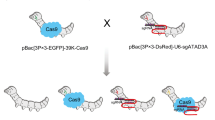
The sericulture industry faces substantial economic losses due to severe pathogenic infections caused by fungi, viruses, and bacteria. The development of transgenic silkworms against specific pathogens has been shown to enhance disease resistance against a particular infection. A single gene or its products that can confer protection against multiple pathogens is required. In an attempt to develop silkworms with enhanced immunity against multiple pathogens, we generated transgenic silkworm lines with an overexpressed NF-kB transcription factor, Relish 1, under two different promoters. Separately, a potent anti-fungal gene, Drosomycin, was also expressed in transgenic silkworms. Both Relish 1 and Drosomycin transgenic silkworms had single copy genomic integration, and their mRNA expression levels were highly increased after infection with silkworm pathogens. The overexpression of the Relish 1 in transgenic silkworms resulted in the upregulation of several defense-related genes, Cecropin B, Attacin, and Lebocin, and showed enhanced resistance to Nosema bombycis (microsporidian fungus), Nucleopolyhedrovirus (BmNPV), and bacteria. The Drosomycin expressing transgenic silkworms showed elevated resistance to N. bombycis and bacteria. These findings demonstrate the role of Relish 1 in long-lasting protection against multiple pathogens in silkworms. Further, the successful introduction of a foreign gene, Drosomycin, also led to improved disease resistance in silkworms.






Similar content being viewed by others
References
Ravikumar, G., Raje Urs, S., Vijaya Prakash, N. B., Rao, C. G., & Vardhana, K. V. (2011). Development of a multiplex polymerase chain reaction for the simultaneous detection of microsporidians, nucleopolyhedrovirus, and densovirus infecting silkworms. Journal of Invertebrate Pathology, 107, 193–197.
Tayal, M. K., & Chauhan, T. P. S. (2017). Silkworm diseases and pests. Industrial entomology (pp. 265–289). Springer.
Xia, Q., Li, S., & Feng, Q. (2014). Advances in silkworm studies accelerated by the genome sequencing of Bombyx mori. Annual Review of Entomology, 59, 513–536.
Jiang, L., & Xia, Q. (2014). The progress and future of enhancing antiviral capacity by transgenic technology in the silkworm Bombyx mori. Insect Biochemistry and Molecular Biology, 48, 1–7.
Jiang, L., Goldsmith, M. R., & Xia, Q. (2021). Advances in the arms race between silkworm and baculovirus. Frontiers in Immunology, 12, 628151.
Kanginakudru, S., Royer, C., Edupalli, S. V., Jalabert, A., Mauchamp, B., Chandrashekaraiah, Prasad, S. V., Chavancy, G., Couble, P., & Nagaraju, J. (2007). Targeting ie-1 gene by RNAi induces baculoviral resistance in Lepidopteran cell lines and in transgenic silkworms. Insect Molecular Biology, 16, 635–644.
Jiang, L., Wang, G., Cheng, T., Yang, Q., Jin, S., Lu, G., Wu, F., Xiao, Y., Xu, H., & Xia, Q. (2012). Resistance to Bombyx mori nucleopolyhedrovirus via overexpression of an endogenous antiviral gene in transgenic silkworms. Archives of Virology, 157, 1323–1328.
Jiang, L., Cheng, T., Zhao, P., Yang, Q., Wang, G., Jin, S., Lin, P., Xiao, Y., & Xia, Q. (2012). Resistance to BmNPV via overexpression of an exogenous gene controlled by an inducible promoter and enhancer in transgenic silkworm Bombyx mori. PLoS ONE, 7, e41838.
Yang, J. G., Liu, T. H., Dong, X. L., Wu, Y. F., Zhang, Q., Zhou, L., Chen, P., Lu, C., & Pan, M. H. (2017). In vivo RNA interference of BmNHR96 enhances the resistance of transgenic silkworm to BmNPV. Biochemical and Biophysical Research Communications, 493, 332–339.
Zhao, P., Xia, F., Jiang, L., Guo, H., Xu, G., Sun, Q., Wang, B., Wang, Y., Lu, Z., & Xia, Q. (2018). Enhanced antiviral immunity against Bombyx mori cytoplasmic polyhedrosis virus via overexpression of peptidoglycan recognition protein S2 in transgenic silkworms. Developmental & Comparative Immunology, 87, 84–89.
Dong, Z., Long, J., Huang, L., Hu, Z., Chen, P., Hu, N., Zheng, N., Huang, X., Lu, C., & Pan, M. (2019). Construction and application of an HSP70 promoter–inducible genome editing system in transgenic silkworm to induce resistance to Nosema bombycis. Applied Microbiology and Biotechnology, 103, 9583–9592.
Jiang, L., Liu, W., Guo, H., Dang, Y., Cheng, T., Yang, W., Sun, Q., Wang, B., Wang, Y., Xie, E., & Xia, Q. (2019). Distinct functions of Bombyx mori peptidoglycan recognition protein 2 in immune responses to bacteria and viruses. Frontiers in Immunology, 10, 776.
Xu, J., Luo, X., Fang, G., Zhan, S., Wu, J., Wang, D., & Huang, Y. (2020). Transgenic expression of antimicrobial peptides from black soldier fly enhance resistance against entomopathogenic bacteria in the silkworm, Bombyx mori. Insect Biochemistry and Molecular Biology, 127, 103487.
Guo, H., Sun, Q., Wang, B., Wang, Y., Xie, E., Xia, Q., & Jiang, L. (2019). Spry is downregulated by multiple viruses to elevate ERK signaling and ensure viral reproduction in silkworm. Development and Comparative Immunology, 98, 1–5.
Jiang, L. (2021). Insights into the antiviral pathways of the silkworm Bombyx mori. Frontiers in Immunology, 12, 639092.
Hedengren, M., Dushay, M. S., Ando, I., Ekengren, S., Wihlborg, M., & Hultmark, D. (1999). Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Molecular Cell, 4, 827–837.
Schluns, H., & Crozier, R. H. (2007). Relish regulates expression of antimicrobial peptide genes in the honeybee, Apis mellifera, shown by RNA interference. Insect Molecular Biology, 16, 753–759.
Antonova, Y., Alvarez, K. S., Kim, Y. J., Kokoza, V., & Raikhel, A. S. (2009). The role of NF-κB factor REL2 in the Aedes aegypti immune response. Insect Biochemistry and Molecular Biology, 39, 303–314.
Lemaitre, B., Reichhart, J. M., & Hoffmann, J. A. (1997). Drosophila host defense: Differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proceedings of the National Academy of Sciences, 94, 14614–14619.
Tanaka, H., Matsuki, H., Furukawa, S., Sagisaka, A., Kotani, E., Mori, H., & Yamakawa, M. (2007). Identification and functional analysis of Relish homologs in the silkworm, Bombyx mori. Biochimica et Biophysica Acta, 1769, 559–568.
Tanaka, H., Ishibashi, J., Fujita, K., Nakajima, Y., Sagisaka, A., Tomimoto, K., Suzuki, N., Yoshiyama, M., Kaneko, Y., Iwasaki, T., & Sunagawa, T., Yamaji, K., Asaoka, A., Mita, K., & Yamakawa, M. (2008). A genome-wide analysis of genes and gene families involved in innate immunity of Bombyx mori. Insect Biochemistry and Molecular Biology, 38, 1087–1110.
Fehlbaum, P., Bulet, P., Michaut, L., Lagueux, M., Broekaert, W. F., Hetru, C., & Hoffmann, J. A. (1994). Insect immunity. Septic injury of Drosophila induces the synthesis of a potent antifungal peptide with sequence homology to plant antifungal peptides. Journal of Biological Chemistry, 269, 33159–33163.
Lemaitre, B., Nicolas, E., Michaut, L., Reichhart, J. M., & Hoffmann, J. A. (1996). The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell, 86, 973–983.
Landon, C., Sodano, P., Hetru, C., Hoffmann, J., & Ptak, M. (1997). Solution structure of Drosomycin, the first inducible antifungal protein from insects. Protein Science, 6, 1878–1884.
Ullal, S., R., & Narasimhanna, M. N. (1994). Hand book of sericulture. In Sampath, J. (Ed). Central Silk Board.
Tamura, T., Thibert, C., Royer, C., Kanda, T., Abraham, E., Kamba, M., Komoto, N., Thomas, J. L., Mauchamp, B., Chavancy, G., Shirk Transgenic Res 123 P, Fraser, M., Prudhomme, J. C., & Couble, P. (2000). Germline transformation of the silkworm Bombyx mori. using a piggyBac transposon-derived vector. Nature Biotechnology, 8, 81–84.
Schmittgen, T. D., & Livak, K. J. (2008). Analyzing real-time PCR data by the comparative CT method. Nature Protocols., 3, 1101–1108.
Handler, A. M., McCombs, S. D., Fraser, M. J., & Saul, S. H. (1998). The Lepidopteran transposon vector, piggyBac, mediates germline transformation in the Mediterranean fruit fly. Proceedings of the National Academy of Sciences, 95, 7520–7525.
Guo, X. Y., Dong, L., Wang, S. P., Guo, T. Q., Wang, J. Y., & Lu, C. D. (2004). Introduction of foreign genes into silkworm eggs by electroporation and its application in transgenic vector test. Acta Biochimia et Biophysia Sinica, 36, 323–330.
Huang, L., Cheng, T., Xu, P., Fang, T., & Xia, Q. (2012). Bombyx mori transcription factors: Genome-wide identification, expression profiles and response to pathogens by microarray analysis. Journal of Insect Science, 12, 1–24.
Li, Z. H., Pan, G. Q., Ma, Z. G., Han, B., Sun, B., Ni, Q., Chen, J., Li, T., Liu, T. B., Long, M. X., Li, C. F., & Zhou, Z. (2017). Comparative proteomic analysis of differentially expressed proteins in the Bombyx mori fat body during the microsporidia, Nosema bombycis infection. Invertebrate Pathology, 149, 36–43.
Hua, X., Li, B., Song, L., Hu, C., Li, X., Wang, D., Xiong, Y., Zhao, P., He, H., Xia, Q., & Wang, F. (2018). Stimulator of interferon genes (STING) provides insect antiviral immunity by promoting Dredd caspase-mediated NF-κB activation. Journal of Biologial Chemistry, 293, 11878–11890.
Hungund, S. P., Pradeep, A. N. R., Makwana, P., Sagar, C., & Mishra, R. K. (2020). Cellular defence and innate immunity in the larval ovarian disc and differentiated ovariole of the silkworm Bombyx mori induced by microsporidian infection. Invertebrate Reprodution and Development, 64, 10–21.
Hua, X., Xu, W., Ma, S., & Xia, Q. (2021). STING-dependent autophagy suppresses Nosema bombycis infection in silkworm, Bombyx mori. Developmental and Comparative Immunology, 115, 103862.
Dong, Y., Das, S., Cirimotich, C., Souza-Neto, J. A., McLean, K. J., & Dimopoulos, G. (2011). Engineered Anopheles immunity to Plasmodium infection. PLoS Pathogen, 7, e1002458.
De Gregorio, E., Spellman, P. T., Tzou, P., Rubin, G. M., & Lemaitre, B. (2002). The Toll and Imd pathways are the major regulators of the immune response in Drosophila. The EMBO Journal, 21, 2568–2579.
Tanaka, H., Sagisaka, A., Nakajima, Y., Fujita, K., Imanishi, S., & Yamakawa, M. (2009). Correlation of differential expression of silkworm antimicrobial peptide genes with different amounts of rel family proteins and their gene transcriptional activity. Bioscience, Biotechnology, and Biochemistry, 73, 599–606.
Keshavarz, M., Jo, Y. H., Park, K. B., Ko, H. J., Edosa, T. T., Lee, Y. S., & Han, Y. S. (2019). Tm DorX2 positively regulates antimicrobial peptides in Tenebrio molitor gut, fat body, and hemocytes in response to bacterial and fungal infection. Scientific Reports, 9, 1–19.
Subbaiah, E. V., Royer, C., Kanginakudru, S., Satyavathi, V. V., Babu, A. S., Sivaprasad, V., Chavancy, G., Darocha, M., Jalabert, A., Mauchamp, B., Basha, I., Couble, P., & Nagaraju, J. (2013). Engineering silkworms for resistance to baculovirus through multigene RNA interference. Genetics, 193, 63–75.
Jiang, L., Peng, Z., Guo, H., Sun, J., Sun, Q., Xia, F., Huang, C., Xu, G., & Xia, Q. (2017). Enhancement of antiviral capacity of transgenic silkworms against cytoplasmic polyhedrosis virus via knockdown of multiple viral genes. Developmental & Comparative Immunology, 77, 138–140.
Sun, Q., Jiang, L., Guo, H., Xia, F., Wang, B., Wang, Y., Xia, Q., & Zhao, P. (2018). Increased antiviral capacity of transgenic silkworm via knockdown of multiple genes on Bombyx mori bidensovirus. Developmental & Comparative Immunology, 87, 188–192.
Valanne, S., Wang, J. H., & Ramet, M. (2011). The Drosophila toll signaling pathway. The Journal of Immunology, 186, 649–656.
Tian, C., Gao, B., del Carmen Rodriguez, M., Lanz-Mendoza, H., Ma, B., & Zhu, S. (2008). Gene expression, antiparasitic activity, and functional evolution of the drosomycin family. Molecular Immunology, 45, 3909–3916.
Keeling, P. (2009). Five questions about microsporidia. PLoS Pathogen, 5, e1000489.
Roxstrom-Lindquist, K., Terenius, O., & Faye, I. (2004). Parasite-specific immune response in adult Drosophila melanogaster: A genomic study. EMBO Reports, 5, 207–212.
Tzou, P., Reichhart, J. M., & Lemaitre, B. (2002). Constitutive expression of a single antimicrobial peptide can restore wild-type resistance to infection in immunodeficient Drosophila mutants. Proceedings of the National Academy of Sciences, 99, 2152–2157.
Michel, T., Reichhart, J. M., Hoffmann, J. A., & Royet, J. (2001). Drosophila Toll is activated by gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature, 414, 756–759.
Ma, Z., Li, C., Pan, G., Li, Z., Han, B., Xu, J., Lan, X., Chen, J., Yang, D., Chen, Q., & Sang, Q. (2013). Genome-wide transcriptional response of silkworm (Bombyx mori) to infection by the microsporidian Nosema bombycis. PLoS ONE, 8, e84137.
Zambon, R. A., Nandakumar, M., Vakharia, V. N., & Wu, L. P. (2005). The Toll pathway is important for an antiviral response in Drosophila. Proceedings of the National Academy of Sciences, 102, 7257–7262.
Acknowledgements
This work was supported by a Grant (No. AIT 3540) to RG and UN from Central Silk Board (CSB), Bengaluru, India. RSY, DST, CM, and GR are thankful to CSB for providing research fellowships.
Author information
Authors and Affiliations
Contributions
RSY performed experiments, data organization and analysis, and writing-first draft. DST, CM, and GR assisted in experiments, data organization and analysis, and writing-review. VK analyzed the data and review. RKM performed funding acquisition, resources and coordination. UN guided in experiments, supervision, writing-review and editing. RG did conceptualization, funding acquisition, guidance in experiments, supervision, and writing-review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rasalkar Sandhya Yashwant—Research Scholar of Jain University, Bengaluru, India under the guidance of RG.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yashwant, R.S., Thomas, D.S., Manoharan, C. et al. Transgenic Silkworms Overexpressing Relish and Expressing Drosomycin Confer Enhanced Immunity to Multiple Pathogens. Mol Biotechnol 64, 711–724 (2022). https://doi.org/10.1007/s12033-021-00438-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-021-00438-0