Abstract
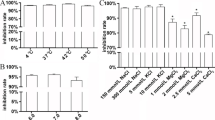
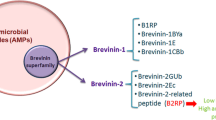
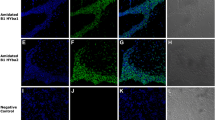
Natural peptides have been the source of some important tools to address challenges in protein therapy of diseases. Bypassing cell plasma membrane has been a bottleneck in the intracellular delivery of biomolecules. Among others, cell-penetrating peptides (CPPs) provide an efficient strategy for intracellular delivery of various cargos. Brevinin-2R peptide is an antimicrobial peptide isolated from the skin secretions of marsh frog, Rana ridibunda with semi-selective anticancer properties. Here, we investigated cell-penetrating properties of Brevinin-2R peptide and its ability to deliver functional protein cargos. Bioinformatics studies showed that Brevinin-2R is a cationic peptide with a net charge of + 5 with an alpha-helix structure and a heptameric ring at the carboxylic terminal due to disulfide bond between C19 and C25 amino acids and a hinge region at A10. To evaluate the ability of this peptide as a CPP, β-galactosidase protein and GFP were transfected into HeLa cells. The entry pathway of the peptide/protein complex into the cell was investigated by inhibiting endocytic pathways at 4 °C. It was observed that Brevinin-2R can efficiently transfer β-galactosidase and GFP with 21% and 90% efficacy, respectively. Brevinin-2R opts for endocytosis pathways to enter cells. The cytotoxicity of this peptide against HeLa cells was studied using MTT assay. The results showed that at the concentration of 131.5 μg/ml of Brevinin-2R peptide, the proliferation of 50% of HeLa cells was inhibited. The results of this study suggest that Brevinin-2R peptide can act as a CPP of natural origin and low cytotoxicity.










Similar content being viewed by others
References
Stewart, M. P., Sharei, A., Ding, X., Sahay, G., Langer, R., & Jensen, K. F. (2016). In vitro and ex vivo strategies for intracellular delivery. Nature, 538(7624), 183–192.
Frankel, A. D., & Pabo, C. O. (1988). Cellular uptake of the tat protein from human immunodeficiency virus. Cell, 55(6), 1189–1193.
Joliot, A., Pernelle, C., Deagostini-Bazin, H., & Prochiantz, A. (1991). Antennapedia homeobox peptide regulates neural morphogenesis. Proceedings of the National Academy of Science USA, 88(5), 1864–1868.
Derossi, D., Joliot, A. H., Chassaing, G., & Prochiantz, A. (1994). The third helix of the Antennapedia homeodomain translocates through biological membranes. The Journal of Biological Chemistry, 269(14), 10444–10450.
Available from: http://crdd.osdd.net/raghava/cppsite/.
de Figueiredo, I.R., Freire. J. M., Flores, L., Veiga, A. S., Castanho, M. A. (2014). Cell-penetrating peptides: A tool for effective delivery in gene-targeted therapies. IUBMB Life.
Avci, F. G., Akbulut, B. S., & Ozkirimli, E. (2018). Membrane active peptides and their biophysical characterization. Biomolecules, 8(3), 77.
Milletti, F. (2012). Cell-penetrating peptides: Classes, origin, and current landscape. Drug Discovery Today, 17(15–16), 850–860.
Radis-Baptista, G., Campelo, I. S., Morlighem, J. R. L., Melo, L. M., & Freitas, V. J. F. (2017). Cell-penetrating peptides (CPPs): From delivery of nucleic acids and antigens to transduction of engineered nucleases for application in transgenesis. Journal of Biotechnology, 252, 15–26.
Wang, F., Wang, Y., Zhang, X., Zhang, W., Guo, S., & Jin, F. (2014). Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. Journal of Controlled Release, 174, 126–136.
Lewis, H. D., Husain, A., Donnelly, R. J., Barlos, D., Riaz, S., Ginjupalli, K., et al. (2010). Creation of a novel peptide with enhanced nuclear localization in prostate and pancreatic cancer cell lines. BMC Biotechnology, 10(1), 79.
Zhang, Y., Song, J., Zhang, W., Liang, R., Ma, Y., Zhang, L., et al. (2014). Functional properties of a novel hybrid antimicrobial peptide NS: Potent antitumor activity and efficient plasmid delivery. Journal of Peptide Science., 20(10), 785–793.
Zhang, W., Song, J., Liang, R., Zheng, X., Chen, J., Li, G., et al. (2013). Stearylated antimicrobial peptide [D]-K6L9 with cell penetrating property for efficient gene transfer. Peptides, 46, 33–39.
Splith, K., & Neundorf, I. (2011). Antimicrobial peptides with cell-penetrating peptide properties and vice versa. European Biophysics Journal: EBJ., 40(4), 387–397.
Nooranian, S., Oskuee, R. K., & Jalili, A. (2021). Antimicrobial peptides, a pool for novel cell penetrating peptides development and vice versa. International Journal of Peptide Research and Therapeutics, 27, 1205–1220.
Morikawa, N., Hagiwara, K., & Nakajima, T. (1992). Brevinin-1 and -2, unique antimicrobial peptides from the skin of the frog, Rana brevipoda porsa. Biochemical and Biophysical Research Communications., 189(1), 184–90.
Ghavami, S., Asoodeh, A., Klonisch, T., Halayko, A. J., Kadkhoda, K., Kroczak, T. J., et al. (2008). Brevinin-2R(1) semi-selectively kills cancer cells by a distinct mechanism, which involves the lysosomal-mitochondrial death pathway. Journal of Cellular and Molecular Medicine, 12(3), 1005–1022.
Ghodsi-Moghadam, B., & Asoodeh, A. (2018). The impact of Brevinin-2R peptide on oxidative statues and antioxidant enzymes in human epithelial cell line of A549. International Journal of Peptide Research and Therapeutics. https://doi.org/10.1007/s10989-018-9754-1
Asoodeh, A., Haghparast, A., Kashef, R., & Chamani, J. (2013). Pro-inflammatory cytokine responses of A549 epithelial cells to antimicrobial peptide brevinin-2R. International Journal of Peptide Research and Therapeutics, 19(2), 157–162.
Zahedifard, F., Lee, H., No, J. H., Salimi, M., Seyed, N., Asoodeh, A., et al. (2019). Anti-leishmanial activity of Brevinin 2R and its lauric acid conjugate type against L. Major: In vitro mechanism of actions and in vivo treatment potentials. PLoS Neglected Tropical Diseases, 4, 3. https://doi.org/10.1371/journal.pntd.0007217
Mehrnejad, F., Naderi-Manesh, H., Ranjbar, B., Maroufi, B., Asoodeh, A., & Doustdar, F. (2008). PCR-based gene synthesis, molecular cloning, high level expression, purification, and characterization of novel antimicrobial peptide, Brevinin-2R, in Escherichia coli. Applied Biochemistry and Biotechnology, 149(2), 109–118.
Yaghoubi, H., Asef, A., & Banki, A. (2013). solid phase chemical synthesis and structure–activity study of brevinin-2R and analogues as antimicrobial peptides. Journal of Medical Bacteriology, 2, 41–53.
Ghorani-Azam, A., Balali-Mood, M., Aryan, E., Karimi, G., & Riahi-Zanjani, B. (2018). Effect of amino acid substitution on biological activity of cyanophlyctin-β and brevinin-2R. Journal of Molecular Structure, 1158, 14–18.
Homayouni-Tabrizi, M., Asoodeh, A., Mashreghi, M., Bazaz, M. R., Oskuee, R. K., & Darroudi, M. (2016). Attachment of a frog skin-derived peptide to functionalized cerium oxide nanoparticles. International Journal of Peptide Research and Therapeutics, 22(4), 505–510.
Jamadi, R. H., Asadi, A., Yaghoubi, H., & Goudarzi, F. (2018). Investigation into the anticancer activity and apoptosis induction of brevinin-2R and brevinin-2R-conjugated PLA–PEG–PLA nanoparticles and strong cell cycle arrest in AGS, HepG2 and KYSE-30 cell lines. International Journal of Peptide Research and Therapeutics. https://doi.org/10.1007/s10989-018-9772-z
Zohrab, F., Asoodeh, A., Jalili, A., Darroudi, M., & KazemiOskuee, R. (2019). Brevinin-2R-linked polyethylenimine as a promising hybrid nano-gene-delivery vector. Iranian Journal of Basic Medical Sciences, 22(9), 1026–35.
Adiyaman, R., & McGuffin, L. J. (2019). Methods for the refinement of protein structure 3D models. International journal of molecular sciences., 20(9), 2301.
He, H., Sun, L., Ye, J., Liu, E., Chen, S., Liang, Q., et al. (2016). Enzyme-triggered, cell penetrating peptide-mediated delivery of anti-tumor agents. Journal of Controlled Release, 240, 67–76.
Patel, S. G., Sayers, E. J., He, L., Narayan, R., Williams, T. L., Mills, E. M., et al. (2019). Cell-penetrating peptide sequence and modification dependent uptake and subcellular distribution of green florescent protein in different cell lines. Scientific Reports, 9(1), 6298.
Morris, M. C., Depollier, J., Mery, J., Heitz, F., & Divita, G. (2001). A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nature Biotechnology, 19, 1173.
Hancock, R. E., & Sahl, H. G. (2006). Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nature Biotechnology, 24(12), 1551–1557.
Huan, Y., Kong, Q., Mou, H., & Yi, H. (2020). Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Frontiers in Microbiology, 11, 2559.
HassanvandJamadi, R., Yaghoubi, H., & Sadeghizadeh, M. (2017). Brevinin-2R and derivatives as potential anticancer peptides: synthesis, purification, characterization and biological activities. International Journal of Peptide Research and Therapeutics. https://doi.org/10.1007/s10989-017-9656-7
HassanvandJamadi, R., Yaghoubi, H., & Sadeghizadeh, M. (2019). Brevinin-2R and derivatives as potential anticancer peptides: synthesis, purification, characterization and biological activities. International Journal of Peptide Research and Therapeutics, 25(1), 151–160.
Turner, J. J., Jones, S., Fabani, M. M., Ivanova, G., Arzumanov, A. A., & Gait, M. J. (2007). RNA targeting with peptide conjugates of oligonucleotides, siRNA and PNA. Blood Cells, Molecules, and Diseases, 38(1), 1–7.
Meade, B. R., & Dowdy, S. F. (2008). Enhancing the cellular uptake of siRNA duplexes following noncovalent packaging with protein transduction domain peptides. Advanced Drug Delivery Reviews, 60(4–5), 530–536.
Jain, A., Yadav, B. K., & Chugh, A. (2015). Marine antimicrobial peptide tachyplesin as an efficient nanocarrier for macromolecule delivery in plant and mammalian cells. The FEBS Journal, 282(4), 732–745.
Kauffman, W. B., Fuselier, T., He, J., & Wimley, W. C. (2015). Mechanism matters: A taxonomy of cell penetrating peptides. Trends in Biochemical Sciences, 40(12), 749–764.
Ramsey, J. D., & Flynn, N. H. (2015). Cell-penetrating peptides transport therapeutics into cells. Pharmacology & Therapeutics, 154, 78–86.
Stewart, M. P., Lorenz, A., Dahlman, J., & Sahay, G. (2016). Challenges in carrier-mediated intracellular delivery: Moving beyond endosomal barriers. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 8(3), 465–478.
Stewart, M. P., Langer, R., & Jensen, K. F. (2018). Intracellular delivery by membrane disruption: Mechanisms, strategies, and concepts. Chemical reviews., 118(16), 7409–7531.
Bechara, C., & Sagan, S. (2013). Cell-penetrating peptides: 20 years later, where do we stand? FEBS Letters, 587(12), 1693–1702.
Acknowledgements
This study was supported by research Grant No. 951791, provided by Mashhad University of Medical Sciences, Mashhad, Iran. This study was part of the first author MSc thesis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nooranian, S., Kazemi Oskuee, R. & Jalili, A. Characterization and Evaluation of Cell-Penetrating Activity of Brevinin-2R: An Amphibian Skin Antimicrobial Peptide. Mol Biotechnol 64, 546–559 (2022). https://doi.org/10.1007/s12033-021-00433-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-021-00433-5