Abstract
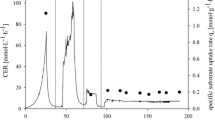
Downstream processing is an expensive step for industrial production of recombinant proteins. Cell immobilization is known as one of the ideal solutions in regard to process intensification. In recent years, magnetic immobilization was introduced as a new technique for cell immobilization. This technique was successfully employed to harvest many bacterial and eukaryotic cells. But there are no data about the influence of magnetic immobilization on the eukaryotic inducted recombinant cells. In this study, impacts of magnetic immobilization on the growth and metabolic status of induced recombinant Pichia pastoris as a valuable eukaryotic model cells were investigated. Results based on colony-forming unit, OD600, and trypan blue assay indicated that magnetic immobilization had no adverse effect on the growth and viability of P. pastoris cells. Also, about 20–40% increase in metabolic activity was recorded in immobilized cells that were decorated with 0.5–2 mg/mL nanoparticles. Total protein and carbohydrate of the cells were also measured as main indicatives for cell function and no significant changes were observed in the immobilized cells. Current data show magnetic immobilization as a biocompatible technique for application in eukaryotic expression systems. Results can be considered for further developments in P. pastoris-based expression systems.










Similar content being viewed by others
References
Daly, R., & Hearn, M. T. (2005). Expression of heterologous proteins in Pichia pastoris: A useful experimental tool in protein engineering and production. Journal of Molecular Recognition, 18, 119–138.
Song, C. P., Liew, P. E., Teh, Z., Lim, S. P., Show, P. L., & Ooi, C. W. (2018). Purification of the recombinant green fluorescent protein using aqueous two-phase system composed of recyclable CO2-based alkyl carbamate ionic liquid. Frontiers in Chemistry, 6, 529.
Wang, Y., Ling, C., Chen, Y., Jiang, X., & Chen, G.-Q. (2019). Microbial engineering for easy downstream processing. Biotechnology Advances, 37, 107365.
Ebrahiminezhad, A., Varma, V., Yang, S., & Berenjian, A. (2016). Magnetic immobilization of Bacillus subtilis natto cells for menaquinone-7 fermentation. Applied Microbiology and Biotechnology, 100, 173–180.
Taghizadeh, S.-M., Berenjian, A., Chew, K. W., Show, P. L., Mohd Zaid, H. F., Ramezani, H., et al. (2020). Impact of magnetic immobilization on the cell physiology of green unicellular algae Chlorella vulgaris. Bioengineered, 11, 141–153.
Raee, M. J., Ebrahiminezhad, A., Gholami, A., Ghoshoon, M. B., & Ghasemi, Y. (2018). Magnetic immobilization of recombinant E. coli producing extracellular asparaginase: An effective way to intensify downstream process. Separation Science and Technology, 53, 1397–1404.
Safarik, I., Maderova, Z., Pospiskova, K., Baldikova, E., Horska, K., & Safarikova, M. (2015). Magnetically responsive yeast cells: Methods of preparation and applications. Yeast, 32, 227–237.
Safarik, I., Pospiskova, K., Maderova, Z., Baldikova, E., Horska, K., & Safarikova, M. (2015). Microwave-synthesized magnetic chitosan microparticles for the immobilization of yeast cells. Yeast, 32, 239–243.
Firoozi, F. R., Raee, M. J., Lal, N., Ebrahiminezhad, A., Teshnizi, S. H., Berenjian, A., et al. (2021). Application of magnetic immboilization for ethanol biosynthesis using Saccharomyces cerevisiae. Separation Science and Technology. https://doi.org/10.1080/01496395.2021.19393761-11
Taghizadeh, S.-M., Ebrahiminezhad, A., Ghoshoon, M. B., Dehshahri, A., Berenjian, A., & Ghasemi, Y. (2020). Magnetic Immobilization of Pichia pastoris cells for the production of recombinant human serum albumin. Nanomaterials, 10, 111.
Peng, Q., Huo, D., Li, H., Zhang, B., Li, Y., Liang, A., et al. (2018). ROS-independent toxicity of Fe3O4 nanoparticles to yeast cells: Involvement of mitochondrial dysfunction. Chemico-Biological Interactions, 287, 20–26.
Otero-González, L., García-Saucedo, C., Field, J. A., & Sierra-Álvarez, R. (2013). Toxicity of TiO2, ZrO2, Fe0, Fe2O3, and Mn2O3 nanoparticles to the yeast, Saccharomyces cerevisiae. Chemosphere, 93, 1201–1206.
Luo, F., Zhu, S., Hu, Y., Yang, K.-C., He, M.-S., Zhu, B., et al. (2020). Biocompatibility assessment of Fe3O4 nanoparticles using Saccharomyces cerevisiae as a model organism. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 227, 108645.
Bhargava, P., & Collins, J. J. (2015). Boosting bacterial metabolism to combat antibiotic resistance. Cell Metabolism, 21, 154–155.
Cabral, D. J., Penumutchu, S., Reinhart, E. M., Zhang, C., Korry, B. J., Wurster, J. I., et al. (2019). Microbial metabolism modulates antibiotic susceptibility within the murine gut microbiome. Cell Metabolism, 30, 800–823.
Stokes, J. M., Lopatkin, A. J., Lobritz, M. A., & Collins, J. J. (2019). Bacterial metabolism and antibiotic efficacy. Cell Metabolism, 30, 251–259.
Ebrahiminezhad, A., Ghasemi, Y., Rasoul-Amini, S., Barar, J., & Davaran, S. (2013). Preparation of novel magnetic fluorescent nanoparticles using amino acids. Colloids and Surfaces B, 102, 534–539.
Liu, P., Wang, X., Hiltunen, K., & Chen, Z. (2015). Controllable drug release system in living cells triggered by enzyme–substrate recognition. ACS Applied Materials & Interfaces, 7, 26811–26818.
Olson, B. J., & Markwell, J. (2007). Assays for determination of protein concentration. Current Protocols in Protein Science. https://doi.org/10.1002/0471140864.ps0471140304s0471140848
Ebrahiminezhad, A., Varma, V., Yang, S., Ghasemi, Y., & Berenjian, A. (2015). Synthesis and application of amine functionalized iron oxide nanoparticles on menaquinone-7 fermentation: A step towards process intensification. Nanomaterials, 6, 1. https://doi.org/10.3390/nano6010001
Li, Y. G., Gao, H. S., Li, W. L., Xing, J. M., & Liu, H. Z. (2009). In situ magnetic separation and immobilization of dibenzothiophene-desulfurizing bacteria. Bioresource Technology, 100, 5092–5096.
Vakili-Ghartavol, R., Momtazi-Borojeni, A. A., Vakili-Ghartavol, Z., Aiyelabegan, H. T., Jaafari, M. R., Rezayat, S. M., et al. (2020). Toxicity assessment of superparamagnetic iron oxide nanoparticles in different tissues. Artificial Cells, Nanomedicine, and Biotechnology, 48, 443–451.
Lin, L., Wu, W., Huang, J., Sun, D., Zhou, Y., Wang, H., et al. (2013). Catalytic gold nanoparticles immobilized on yeast: From biosorption to bioreduction. Chemical Engineering Journal, 225, 857–864.
Grumezescu, A. M., Mihaiescu, D. E., Mogosanu, D. E., Chifiriuc, M. C., Lazar, V., Calugarescu, I., et al. (2010). In vitro assay of the antimicrobial activity of Fe3O4 and CoFe2O4/oleic acid—Core/shell on clinical isolates of bacterial and fungal strains. Journal of Optoelectronics and Advanced Materials, 4, 1798–1801.
Ramteke, C., Ketan Sarangi, B., Chakrabarti, T., Mudliar, S., Satpute, D., & Avatar Pandey, R. (2010). Synthesis and broad spectrum antibacterial activity of magnetite ferrofluid. Current Nanoscience, 6, 587–591.
Gholami, A., Rasoul-Amini, S., Ebrahiminezhad, A., Abootalebi, N., Niroumand, U., Ebrahimi, N., et al. (2016). Magnetic properties and antimicrobial effect of amino and lipoamino acid coated iron oxide nanoparticles. Minerva Biotecnologica, 28, 177–186.
Villanueva-Flores, F., Castro-Lugo, A., Ramírez, O. T., & Palomares, L. A. (2020). Understanding cellular interactions with nanomaterials: Towards a rational design of medical nanodevices. Nanotechnology, 31, 132002.
Helmlinger, J., Sengstock, C., Groß-Heitfeld, C., Mayer, C., Schildhauer, T., Köller, M., et al. (2016). Silver nanoparticles with different size and shape: Equal cytotoxicity, but different antibacterial effects. RSC Advances, 6, 18490–18501.
Zhang, B., Lung, P. S., Zhao, S., Chu, Z., Chrzanowski, W., & Li, Q. (2017). Shape dependent cytotoxicity of PLGA-PEG nanoparticles on human cells. Science and Reports, 7, 7315.
Steckiewicz, K. P., Barcinska, E., Malankowska, A., Zauszkiewicz-Pawlak, A., Nowaczyk, G., Zaleska-Medynska, A., et al. (2019). Impact of gold nanoparticles shape on their cytotoxicity against human osteoblast and osteosarcoma in in vitro model. Evaluation of the safety of use and anti-cancer potential. Journal of Materials Science: Materials in Medicine, 30, 22. https://doi.org/10.1007/s10856-10019-16221-10852
Pichia expression kit, Protein expression, A manual of methods for expression of recombinant proteins in Pichia pastoris. Corporation, I., Ed. Invitrogen Corporation: San Diego, CA, USA, Vol. version F.
Matsuo, M., Oogai, Y., Kato, F., Sugai, M., & Komatsuzawa, H. (2011). Growth-phase dependence of susceptibility to antimicrobial peptides in Staphylococcus aureus. Microbiology (Russ. Acad. Sci.), 157, 1786–1797.
Sinclair, P., Carballo-Pacheco, M., & Allen, R. J. (2019). Growth-dependent drug susceptibility can prevent or enhance spatial expansion of a bacterial population. Physical Biology, 16, 046001.
Patel, J. B., Cockerill, F., & Bradford, P. A. (2015). Performance standards for antimicrobial susceptibility testing: Twenty-fifth informational supplement (pp. 29–50). Clinical and Laboratory Standard Institute.
Nazemidashtarjandi, S., & Farnoud, A. M. (2019). Membrane outer leaflet is the primary regulator of membrane damage induced by silica nanoparticles in vesicles and erythrocytes. Environmental Science. Nano, 6, 1219–1232.
Karlsson, H. L., Gustafsson, J., Cronholm, P., & Möller, L. (2009). Size-dependent toxicity of metal oxide particles—A comparison between nano- and micrometer size. Toxicology Letters, 188, 112–118.
Ansari, F., Grigoriev, P., Libor, S., Tothill, I. E., & Ramsden, J. J. (2009). DBT degradation enhancement by decorating Rhodococcus erythropolis IGST8 with magnetic Fe3O4 nanoparticles. Biotechnology and Bioengineering, 102, 1505–1512.
Berovic, M., Berlot, M., Kralj, S., & Makovec, D. (2014). A new method for the rapid separation of magnetized yeast in sparkling wine. Biochemical Engineering Journal, 88, 77–84.
Nocon, J., Steiger, M. G., Pfeffer, M., Sohn, S. B., Kim, T. Y., Maurer, M., et al. (2014). Model based engineering of Pichia pastoris central metabolism enhances recombinant protein production. Metabolic Engineering, 24, 129–138.
Heyland, J., Fu, J., Blank, L. M., & Schmid, A. (2011). Carbon metabolism limits recombinant protein production in Pichia pastoris. Biotechnology and Bioengineering, 108, 1942–1953.
Zahrl, R. J., Peña, D. A., Mattanovich, D., & Gasser, B. (2017). Systems biotechnology for protein production in Pichia pastoris. FEMS Yeast Research, 17, fox068. https://doi.org/10.1093/femsyr/fox1068
Acknowledgements
This experiment was funded by Shiraz University of Medical Sciences, under a PhD thesis proposal submitted at No. 18588 in the School of Pharmacy. Authors are grateful to the support provided by the University of Waikato, New Zealand.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest which influence this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tagizadeh, SM., Ebrahiminezhad, A., Ghoshoon, M.B. et al. Impacts of Magnetic Immobilization on the Growth and Metabolic Status of Recombinant Pichia pastoris. Mol Biotechnol 64, 320–329 (2022). https://doi.org/10.1007/s12033-021-00420-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-021-00420-w