Abstract
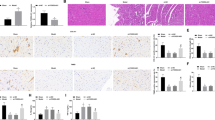
Tendon adhesion is the biggest obstacle to repair of tendon injury. Long-chain non-coding RNA X-inactive specific transcript (lncRNA XIST) is highly expressed in populations at high risk of tendon injury. However, whether XIST participates in tendon injury and the specific mechanism remain unknown. Here, we aimed to explore the effects and underlying mechanism of XIST in tendon injury. A mouse model of tendon injury was constructed by the transection method in vivo. XIST and COX2 were highly expressed in tendon tissues of mice with tendon injury, while miR-26a-5p was lowly expressed. Fibroblasts were isolated from tendon injury mice. Overexpression of XIST promoted fibroblast proliferation and upregulated α-SMA and Collagen I protein expression, while silencing XIST indicated the opposite effects. Further dual-luciferase reporter gene assay and RIP assay verified a targeting relationship between XIST and miR-26a-5p, as well as miR-26a-5p and COX2, and XIST targeted miR-26a-5p to act on COX2 expression. miR-26a-5p inhibition and COX2 overexpression reversed the decrease in fibroblast proliferation and the downregulation of α-SMA and Collagen I expression caused by XIST silencing, while interference with si-COX2 eliminated the effects of miR-26a-5p inhibitor. This study revealed that XIST promoted fibroblast proliferation and the formation of tendon adhesion through miR-26a-5p/COX2 pathway, suggesting that XIST/miR-26a-5p/COX2 may be a potential target for the treatment of tendon injury.




Similar content being viewed by others
References
Legrand, A., et al. (2017). Molecular biology of flexor tendon healing in relation to reduction of tendon adhesions. Journal of Hand Surgery (American Volume), 42(9), 722–726.
Ishiyama, N., et al. (2010). The prevention of peritendinous adhesions by a phospholipid polymer hydrogel formed in situ by spontaneous intermolecular interactions. Biomaterials, 31(14), 4009–4016.
Tang, J. B., et al. (2016). Gene therapy strategies to improve strength and quality of flexor tendon healing. Expert Opinion on Biological Therapy, 16(3), 291–301.
Wang, J.H., Q. Guo, & B. Li. (2012) Tendon biomechanics and mechanobiology--a minireview of basic concepts and recent advancements. The Journal of Hand Therapy, 25(2), 133–40; quiz 141.
Jiang, S., et al. (2014). Down-regulating ERK1/2 and SMAD2/3 phosphorylation by physical barrier of celecoxib-loaded electrospun fibrous membranes prevents tendon adhesions. Biomaterials, 35(37), 9920–9929.
Ruan, H., et al. (2013). Prevention of tendon adhesions by ERK2 small interfering RNAs. International Journal of Molecular Sciences, 14(2), 4361–4371.
Jamil, S., et al. (2017). Angiopoietin-like 4 enhances the proliferation and migration of tendon fibroblasts. Medicine and Science in Sports and Exercise, 49(9), 1769–1777.
Pan, Z., et al. (2020). Upregulation of HSP72 attenuates tendon adhesion by regulating fibroblast proliferation and collagen production via blockade of the STAT3 signaling pathway. Cell Signal. 71, 109606.
Korbecki, J., et al. (2015). Vanadium compounds as pro-inflammatory agents: effects on cyclooxygenases. International Journal of Molecular Sciences, 16(6), 12648–12668.
Mbonye, U. R., & Song, I. (2009). Posttranscriptional and posttranslational determinants of cyclooxygenase expression. BMB Reports, 42(9), 552–560.
Chen, S., et al. (2017) RelA/p65 inhibition prevents tendon adhesion by modulating inflammation, cell proliferation, and apoptosis. Cell Death and Disease, 8(3), e2710.
Zhou, Y. L., et al. (2018). Localized delivery of miRNAs targets cyclooxygenases and reduces flexor tendon adhesions. Acta Biomaterialia, 70, 237–248.
Wang, B., et al. (2016). MiR124 suppresses collagen formation of human tendon derived stem cells through targeting egr1. Experimental Cell Research, 347(2), 360–366.
Chen, Q., Lu, H., & Yang, H. (2014). Chitosan inhibits fibroblasts growth in Achilles tendon via TGF-β1/Smad3 pathway by miR-29b. International Journal of Clinical and Experimental Pathology, 7(12), 8462–8470.
Liang, L., et al. (2017). Down-regulation of miR-26a-5p in hepatocellular carcinoma: a qRT-PCR and bioinformatics study. Pathology, Research and Practice, 213(12), 1494–1509.
Song, Q., et al. (2018). MiR-26a-5p potentiates metastasis of human lung cancer cells by regulating ITGβ8- JAK2/STAT3 axis. Biochemical and Biophysical Research Communications, 501(2), 494–500.
Zheng, L., Lin, S., & Lv, C. (2018). MiR-26a-5p regulates cardiac fibroblasts collagen expression by targeting ULK1. Science and Reports, 8(1), 2104.
Thankam, F. G., et al. (2016). MicroRNAs associated with shoulder tendon matrisome disorganization in glenohumeral arthritis. PLoS ONE, 11(12), e0168077.
Kwon, Y., et al. (2015). MicroRNA-26a/-26b-COX-2-MIP-2 loop regulates allergic inflammation and allergic inflammation-promoted enhanced tumorigenic and metastatic potential of cancer cells. Journal of Biological Chemistry, 290(22), 14245–14266.
Zhang, Q., et al. (2015). Microarray profiling analysis of long non-coding RNAs expression in tendinopathy: Identification for potential biomarkers and mechanisms. International Journal of Experimental Pathology, 96(6), 387–394.
Lu, Y. F., et al. (2017). Long noncoding RNA H19 accelerates tenogenic differentiation and promotes tendon healing through targeting miR-29b-3p and activating TGF-β1 signaling. The FASEB Journal, 31(3), 954–964.
Wang, Z., et al. (2017). Long non-coding RNA XIST exerts oncogenic functions in human glioma by targeting miR-137. American Journal of Translational Research, 9(4), 1845–1855.
Sun, Z., Zhang, B., & Cui, T. (2018). Long non-coding RNA XIST exerts oncogenic functions in pancreatic cancer via miR-34a-5p. Oncology Reports, 39(4), 1591–1600.
Yuan, X., et al. (2021). METTL3 regulates ossification of the posterior longitudinal ligament via the lncRNA XIST/miR-302a-3p/USP8 Axis. Frontiers in Cell and Developmental Biology, 9, 629895.
Cao, W., & Feng, Y. (2019). LncRNA XIST promotes extracellular matrix synthesis, proliferation and migration by targeting miR-29b-3p/COL1A1 in human skin fibroblasts after thermal injury. Biological Research, 52(1), 52.
Peffers, M. J., et al. (2015). Transcriptome analysis of ageing in uninjured human Achilles tendon. Arthritis Research & Therapy, 17(1), 33.
Yan, X. T., et al. (2018). XIST accelerates neuropathic pain progression through regulation of miR-150 and ZEB1 in CCI rat models. Journal of Cellular Physiology, 233(8), 6098–6106.
Yu, Y., et al. (2018). Knockdown of lncRNA KCNQ1OT1 suppresses the adipogenic and osteogenic differentiation of tendon stem cell via downregulating miR-138 target genes PPARγ and RUNX2. Cell Cycle, 17(19–20), 2374–2385.
Yao, Z., et al. (2020). MicroRNA-21-3p engineered umbilical cord stem cell-derived exosomes inhibit tendon adhesion. Journal of Inflammation Research, 13, 303–316.
Milewska, M., et al. (2020). Copper does not induce tenogenic differentiation but promotes migration and increases lysyl oxidase activity in adipose-derived mesenchymal stromal cells. Stem Cells Int., 2020, 9123281.
Tajbakhsh, S. (2017). lncRNA-encoded polypeptide SPAR(s) with mTORC1 to regulate skeletal muscle regeneration. Cell Stem Cell, 20(4), 428–430.
Matsumoto, A., et al. (2017). mTORC1 and muscle regeneration are regulated by the LINC00961-encoded SPAR polypeptide. Nature, 541(7636), 228–232.
Liu, Y., et al. (2018). Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. The Biochemical Journal, 475(22), 3629–3638.
Liu, Y., et al. (2018). MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle, 17(21–22), 2411–2422.
Zhao, Y., et al. (2019) Non-coding RNA regulates the myogenesis of skeletal muscle satellite cells, injury repair and diseases. Cells. 8(9).
Daniels, R., et al. (1997). XIST expression in human oocytes and preimplantation embryos. American Journal of Human Genetics, 61(1), 33–39.
Arnold, A. P. (2017). A general theory of sexual differentiation. Journal of Neuroscience Research, 95(1–2), 291–300.
Gayen, S., et al. (2016). Sex-specific silencing of X-linked genes by Xist RNA. Proceedings of the National Academy of Sciences of the United States of America, 113(3), E309–E318.
Cui, H., et al. (2019). Macrophage-derived miRNA-containing exosomes induce peritendinous fibrosis after tendon injury through the miR-21-5p/Smad7 pathway. Molecular Therapy - Nucleic Acids, 14, 114–130.
Dolkart, O., et al. (2014). Statins enhance rotator cuff healing by stimulating the COX2/PGE2/EP4 pathway: An in vivo and in vitro study. American Journal of Sports Medicine, 42(12), 2869–2876.
Funding
This work was supported by the Zhejiang Medicine and Hygiene Research Program (Grant Number 2019KY025) and Zhejiang Traditional Chinese Medicine Research Program (Grant Number 2019ZA008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Q., Hou, D., Suo, Y. et al. LncRNA XIST Prevents Tendon Adhesion and Promotes Tendon Repair Through the miR-26a-5p/COX2 Pathway. Mol Biotechnol 64, 424–433 (2022). https://doi.org/10.1007/s12033-021-00419-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-021-00419-3