Abstract
This study aimed to examine the protective effects of catalpa on ultrastructure of hippocampal neuron and limb motor function in rats with cerebral ischemia. 90 healthy Sprague–Dawley male rats were randomly divided into control (n = 30) and model (n = 60) groups. Cerebral ischemia and hippocampal neurons were induced by occluding the internal carotid artery and injection of high blood glucose, respectively. Model rats were randomly divided into routine (n = 30) and observational (n = 30) groups. Animals in the routine group received edaravone injection (7 mg/kg/day) for 14 days, while rats in the observation group were treated with catalpol (30 mg/kg/day) for 14 days. Limb motor function score, fine motion execution capability, number of hippocampal neurons retained, and the ultrastructure of hippocampal nerve cells were considered at 3, 7, and 14 days after treatments. A significant difference was observed in the mean scores of limb motor function, fine motor execution ability, and the number of hippocampal neurons retained between groups (p < 0.001). Repetitive treatments with catalpol significantly improved the mean number of hippocampal neurons retained (p < 0.01), limb motor function (p < 0.001), and fine motor execution ability scores (p < 0.01) at 3, 7, and 14 days compared to edaravone. Catalpol treatments also improved the ultrastructure morphology of neuronal cells. Catalpa can effectively improve limb motor function and protect hippocampal neuron function in rats with cerebral ischemia.

Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SD:
-
Sprague–Dawley
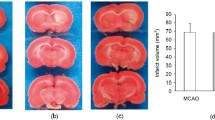
- MCAO:
-
Middle cerebral artery occlusion
- SLMF:
-
Scores of limb motor function
- DIGC:
-
The differences of intra-group comparison
References
Zhao, G. C., Yuan, Y. L., Chai, F. R., & Ji, F. J. (2017). Effect of Melilotus officinalis extract on the apoptosis of brain tissues by altering cerebral thrombosis and inflammatory mediators in acute cerebral ischemia. Biomedicine and pharmacotherapy = Biomedecine & pharmacotherapie, 89, 1346–1352.
Nishibori, M., Mori, S., & Takahashi, H. K. (2019). Anti-HMGB1 monoclonal antibody therapy for a wide range of CNS and PNS diseases. Journal of Pharmacological Sciences, 140(1), 94–101.
Yan, J., Wang, C., Jin, Y., Meng, Q., Liu, Q., Liu, Z., et al. (2018). Catalpol ameliorates hepatic insulin resistance in type 2 diabetes through acting on AMPK/NOX4/PI3K/AKT pathway. Pharmacological Research, 130, 466–480.
Zhu, H. F., Shao, Y., Qin, L., Wang, J. H., Feng, S., Jiang, Y. B., et al. (2019). Catalpol enhances neurogenesis and inhibits apoptosis of new neurons via BDNF, but not the BDNF/Trkb pathway. Drug Design, Development and Therapy, 13, 4145–4157.
Zhang, Y., Wang, C., Jin, Y., Yang, Q., Meng, Q., Liu, Q., et al. (2018). Activating the PGC-1α/TERT pathway by catalpol ameliorates atherosclerosis via modulating ROS production, DNA damage, and telomere function: Implications on mitochondria and telomere link. Oxidative Medicine and Cellular Longevity, 2018, 2876350.
Wan, D., Zhu, H., Luo, Y., & Xie, P. (2011). Changes in synapse quantity and growth associated protein 43 expression in the motor cortex of focal cerebral ischemic rats following catalpol treatment. Neural Regeneration Research, 6(18), 1380–1385.
Wan, D., Xue, L., Zhu, H., & Luo, Y. (2013). Catalpol induces neuroprotection and prevents memory dysfunction through the cholinergic system and BDNF. Evidence-Based Complementary and Alternative Medicine, 2013, 134852.
Dinda, B., Dinda, M., Kulsi, G., Chakraborty, A., & Dinda, S. (2019). Therapeutic potentials of plant iridoids in Alzheimer’s and Parkinson’s diseases: A review. European Journal of Medicinal Chemistry, 169, 185–199.
Zhang, X. L., An, L. J., Bao, Y. M., Wang, J. Y., & Jiang, B. (2008). d-Galactose administration induces memory loss and energy metabolism disturbance in mice: Protective effects of catalpol. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 46(8), 2888–2894.
Zhu, H.-F., Wan, D., Luo, Y., Zhou, J.-L., Chen, L., & Xu, X.-Y. (2010). Catalpol increases brain angiogenesis and up-regulates VEGF and EPO in the rat after permanent middle cerebral artery occlusion. International Journal of Biological Sciences, 6(5), 443–453.
Wan, D., Zhu, H. F., Luo, Y., Xie, P., & Xu, X. Y. (2007). Study of catalpol promoting axonal growth for cultured cortical neurons from rats. Zhongguo zhongyao zazhi China = Journal of Chinese Materia Medica, 32(17), 1771–1774.
Tian, Y. Y., An, L. J., Jiang, L., Duan, Y. L., Chen, J., & Jiang, B. (2006). Catalpol protects dopaminergic neurons from LPS-induced neurotoxicity in mesencephalic neuron-glia cultures. Life Sciences, 80(3), 193–199.
Mao, Y. R., Jiang, L., Duan, Y. L., An, L. J., & Jiang, B. (2007). Efficacy of catalpol as protectant against oxidative stress and mitochondrial dysfunction on rotenone-induced toxicity in mice brain. Environmental Toxicology and Pharmacology, 23(3), 314–318.
Wang, L. Y., Yu, X., Li, X. X., Zhao, Y. N., Wang, C. Y., Wang, Z. Y., et al. (2019). Catalpol exerts a neuroprotective effect in the MPTP mouse model of Parkinson’s disease. Frontiers in Aging Neuroscience, 11, 316.
Liu, C., Ma, R., Wang, L., Zhu, R., Liu, H., Guo, Y., et al. (2017). Rehmanniae Radix in osteoporosis: A review of traditional Chinese medicinal uses, phytochemistry, pharmacokinetics and pharmacology. Journal of Ethnopharmacology, 198, 351–362.
Xu, P., Su, S., Tan, C., Lai, R. S., & Min, Z. S. (2017). Effects of aqueous extracts of Ecliptae herba, Polygoni multiflori radix praeparata and Rehmanniae radix praeparata on melanogenesis and the migration of human melanocytes. Journal of Ethnopharmacology, 195, 89–95.
Jiang, R., Xu, X. H., Wang, K., Yang, X. Z., Bi, Y. F., Yan, Y., et al. (2017). Ethyl acetate extract from Panax ginseng C.A. Meyer and its main constituents inhibit α-melanocyte-stimulating hormone-induced melanogenesis by suppressing oxidative stress in B16 mouse melanoma cells. Journal of Ethnopharmacology, 208, 149–156.
Yang, T., Zheng, Q., Wang, S., Fang, L., Liu, L., Zhao, H., et al. (2017). Effect of catalpol on remyelination through experimental autoimmune encephalomyelitis acting to promote Olig1 and Olig2 expressions in mice. BMC Complementary and Alternative Medicine, 17(1), 240.
Farbiszewski, R., Bielawski, K., Bielawska, A., & Sobaniec, W. (1995). Spermine protects in vivo the antioxidant enzymes in transiently hypoperfused rat brain. Acta neurobiologiae experimentalis, 55(4), 253–258.
Zhang, S., Song, X.-Y., Xia, C.-Y., Ai, Q.-D., Chen, J., Chu, S.-F., et al. (2016). Effects of cerebral glucose levels in infarct areas on stroke injury mediated by blood glucose changes. RSC Advances, 6(96), 93815–93825.
Hausser, N., Johnson, K., Parsley, M. A., Guptarak, J., Spratt, H., & Sell, S. L. (2018). Detecting behavioral deficits in rats after traumatic brain injury. JoVE, 131, e56044.
Mychasiuk, R., Gibb, R., & Kolb, B. (2013). Visualizing the effects of a positive early experience, tactile stimulation, on dendritic morphology and synaptic connectivity with Golgi-Cox staining. JoVE, 79, e50694.
Wang, J., Wan, D., Wan, G., Wang, J., Zhang, J., & Zhu, H. (2019). Catalpol induces cell activity to promote axonal regeneration via the PI3K/AKT/mTOR pathway in vivo and in vitro stroke model. Annals of Translational Medicine, 7(23), 756.
Zheng, X.-W., Yang, W.-T., Chen, S., Xu, Q.-Q., Shan, C.-S., Zheng, G.-Q., et al. (2017). Neuroprotection of catalpol for experimental acute focal ischemic stroke: Preclinical evidence and possible mechanisms of antioxidation, anti-inflammation, and antiapoptosis. Oxidative Medicine and Cellular Longevity, 2017, 5058609.
Huang, J. Z., Wu, J., Xiang, S., Sheng, S., Jiang, Y., Yang, Z., et al. (2016). Catalpol preserves neural function and attenuates the pathology of Alzheimer’s disease in mice. Molecular Medicine Reports, 13(1), 491–496.
Xia, H., Wang, D., Guo, X., Wu, K., Huang, F., & Feng, Y. (2021). Catalpol protects against spinal cord injury in mice through regulating microRNA-142-mediated HMGB1/TLR4/NF-κB signaling pathway. Frontiers in Pharmacology. https://doi.org/10.3389/fphar.2020.630222
Xu, D., Zhao, L., Jiang, J., Li, S., Sun, Z., Huang, X., et al. (2020). A potential therapeutic effect of catalpol in Duchenne muscular dystrophy revealed by binding with TAK1. Journal of Cachexia, Sarcopenia and Muscle, 11(5), 1306–1320.
Acknowledgements
This work was supported by the Key Project of University Research Fund of Xinjiang Uygur Autonomous Region (XJEDU2021I018).
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SX, YZ, and BZ conceived and designed the experiments, performed the experiments and analyzed the data, and drafted the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
All experiments were performed according to standard protocols, incompliance with the Guide of the Animal Ethics Committee of Xinjiang Medical University.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xie, S., Zhi, Y. & Zeng, B. Protective Effects of Catalpol on Limb Motor Function and Ultrastructure of Hippocampal Neurons in Rats with Cerebral Ischemia. Mol Biotechnol 64, 213–219 (2022). https://doi.org/10.1007/s12033-021-00407-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-021-00407-7