Abstract
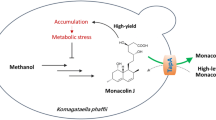
Lovastatin is an anti-cholesterol medicine that is commonly prescribed to manage cholesterol levels, and minimise the risk of suffering from heart-related diseases. Aspergillus terreus (ATCC 20542) supplied with carbohydrates or sugar alcohols can produce lovastatin. The present work explored the application of metabolic engineering in A. terreus to re-route the precursor flow towards the lovastatin biosynthetic pathway by simultaneously overexpressing the gene for acetyl-CoA carboxylase (acc) to increase the precursor flux, and eliminate ( +)-geodin biosynthesis (a competing secondary metabolite) by removing the gene for emodin anthrone polyketide synthase (gedC). Alterations to metabolic flux in the double mutant (gedCΔ*accox) strain and the effects of using two different substrate formulations were examined. The gedCΔ*accox strain, when cultivated with a mixture of glycerol and lactose, significantly (p < 0.05) increased the levels of metabolic precursors malonyl-CoA (48%) and acetyl-CoA (420%), completely inhibited the (+)-geodin biosynthesis, and increased the level of lovastatin [152 mg/L; 143% higher than the wild-type (WT) strain]. The present work demonstrated how the manipulation of A. terreus metabolic pathways could increase the efficiency of carbon flux towards lovastatin, thus elevating its overall production and enabling the use of glycerol as a substrate source. As such, the present work also provides a framework model for other medically or industrially important fungi to synthesise valuable compounds using sustainable carbon sources.




Similar content being viewed by others
Data Availability
The datasets generated and/or analysed in the present work are available from the corresponding author upon reasonable request.
Abbreviations
- PKS:
-
Polyketide synthase
- LNKS:
-
Lovastatin nonaketide synthase
- KS:
-
Ketoacyl synthase
- AT:
-
Acyl transferase
- PDA:
-
Potato dextrose agar
- G:
-
Glycerol
- GL:
-
Glycerol–lactose
- HPLC:
-
High-performance liquid chromatography
- WT:
-
Wild-type
References
Barrios-González, J., & Miranda, R. U. (2010). Biotechnological production and applications of statins. Applied Microbiology and Biotechnology, 85(4), 869–883. https://doi.org/10.1007/s00253-009-2239-6.
Maron, D. J., Fazio, S., & Linton, M. F. (2000). Current perspectives on statins. Circulation, 101(2), 207–213. http://www.ncbi.nlm.nih.gov/pubmed/10637210.
Pecyna, M., & Bizukojc, M. (2011). Lovastatin biosynthesis by Aspergillus terreus with the simultaneous use of lactose and glycerol in a discontinuous fed-batch culture. Journal of Biotechnology, 151(1), 77–86. https://doi.org/10.1016/j.jbiotec.2010.10.079.
Bizukojc, M., & Pecyna, M. (2011). Lovastatin and (+)-geodin formation by Aspergillus terreus ATCC 20542 in a batch culture with the simultaneous use of lactose and glycerol as carbon sources. Engineering in Life Sciences, 11(3), 272–282. https://doi.org/10.1002/elsc.201000179.
Boruta, T., & Bizukojc, M. (2017). Production of lovastatin and itaconic acid by Aspergillus terreus: A comparative perspective. World Journal of Microbiology and Biotechnology, 33(2), 1–12. https://doi.org/10.1007/s11274-017-2206-9.
Goswami, S., Vidyarthi, A. S., Bhunia, B., & Mandal, T. (2012). A review on lovastatin and its production. Journal of Biochemical Technology, 4(1), 581–587.
Casas López, J. L., Sánchez Pérez, J. A., Fernández Sevilla, J. M., Acién Fernández, F. G., Molina Grima, E., & Chisti, Y. (2003). Production of lovastatin by Aspergillus terreus: Effects of the C: N ratio and the principal nutrients on growth and metabolite production. Enzyme and Microbial Technology, 33(2–3), 270–277. https://doi.org/10.1016/S0141-0229(03)00130-3.
Abd Rahim, M. H., Lim, E. J., Hasan, H., & Abbas, A. (2019). The investigation of media components for optimal metabolite production of Aspergillus terreus ATCC 20542. Journal of Microbiological Methods, 164(June), 105672. https://doi.org/10.1016/j.mimet.2019.105672.
Couch, R. D., & Gaucher, G. M. (2004). Rational elimination of Aspergillus terreus sulochrin production. Journal of Biotechnology, 108(2), 171–178. https://doi.org/10.1016/j.jbiotec.2003.10.021.
Nielsen, M. T., Nielsen, J. B., Anyaogu, D. C., Holm, D. K., Nielsen, K. F., Larsen, T. O., & Mortensen, U. H. (2013). Heterologous reconstitution of the intact geodin gene cluster in Aspergillus nidulans through a simple and versatile PCR based approach. PLoS ONE, 8(8), e72871. https://doi.org/10.1371/journal.pone.0072871.
Abd Rahim, M. H., Hasan, H., Montoya, A., & Abbas, A. (2015). Lovastatin and (+)-geodin production by Aspergillus terreus from crude glycerol. Engineering in Life Sciences, 15(2), 220–228. https://doi.org/10.1002/elsc.201400140.
Campbell, C. D., & Vederas, J. C. (2010). Biosynthesis of lovastatin and related metabolites formed by fungal iterative PKS enzymes. Biopolymers, 93(9), 755–763. https://doi.org/10.1002/bip.21428.
Barriuso, J., Nguyen, D. T., Li, J. W.-H., Roberts, J. N., MacNevin, G., Chaytor, J. L., … Ro, D.-K. (2011). Double oxidation of the cyclic nonaketide dihydromonacolin L to monacolin J by a single cytochrome P450 monooxygenase, LovA. Journal of the American Chemical Society, 133(21), 8078–8081. https://doi.org/10.1021/ja201138v.
Ma, S. M., & Tang, Y. (2007). Biochemical characterization of the minimal polyketide synthase domains in the lovastatin nonaketide synthase LovB. The FEBS Journal, 274(11), 2854–2864. https://doi.org/10.1111/j.1742-4658.2007.05818.x.
Askenazi, M., Driggers, E. M., Holtzman, D. A., Norman, T. C., Iverson, S., Zimmer, D. P., … Madden, K. T. (2003). Integrating transcriptional and metabolite profiles to direct the engineering of lovastatin-producing fungal strains. Nature Biotechnology, 21(2), 150–156. https://doi.org/10.1038/nbt781.
Bizukojc, M., & Ledakowicz, S. (2009). Physiological, morphological and kinetic aspects of lovastatin biosynthesis by Aspergillus terreus. Biotechnology Journal, 4(5), 647–664. https://doi.org/10.1002/biot.200800289.
Abd Rahim, M. H., Hasan, H., Lim, E. J., Samrani, P. K., & Abbas, A. (2019). Pretreatment strategies to improve crude glycerol utilisation and metabolite production by Aspergillus terreus. International Journal of Chemical Engineering, 2019, 1–6. https://doi.org/10.1155/2019/2504540.
Caspeta, L., & Nielsen, J. (2013). Toward systems metabolic engineering of Aspergillus and Pichia species for the production of chemicals and biofuels. Biotechnology Journal, 8(5), 534–544. https://doi.org/10.1002/biot.201200345.
Brown, S. H., Bashkirova, L., Berka, R., Chandler, T., Doty, T., McCall, K., … Berry, A. (2013). Metabolic engineering of Aspergillus oryzae NRRL 3488 for increased production of l-malic acid. Applied Microbiology and Biotechnology, 97(20), 8903–8912. https://doi.org/10.1007/s00253-013-5132-2.
Blumhoff, M. L., Steiger, M. G., Mattanovich, D., & Sauer, M. (2013). Targeting enzymes to the right compartment: Metabolic engineering for itaconic acid production by Aspergillus niger. Metabolic Engineering, 19, 26–32. https://doi.org/10.1016/j.ymben.2013.05.003.
Chen, Y., Daviet, L., Schalk, M., Siewers, V., & Nielsen, J. (2013). Establishing a platform cell factory through engineering of yeast acetyl-CoA metabolism. Metabolic Engineering, 15, 48–54. https://doi.org/10.1016/j.ymben.2012.11.002.
Zhang, B., Skory, C. D., & Yang, S.-T. (2012). Metabolic engineering of Rhizopus oryzae: Effects of overexpressing pyc and pepc genes on fumaric acid biosynthesis from glucose. Metabolic Engineering, 14(5), 512–520. https://doi.org/10.1016/j.ymben.2012.07.001.
Hasan, H., Abd Rahim, M. H., Campbell, L., Carter, D., Abbas, A., & Montoya, A. (2018). Overexpression of acetyl-CoA carboxylase in Aspergillus terreus to increase lovastatin production. New Biotechnology, 44(December 2017), 64–71. https://doi.org/10.1016/j.nbt.2018.04.008.
Hasan, H., Abd Rahim, M. H., Campbell, L., Carter, D., Abbas, A., & Montoya, A. (2019). Improved lovastatin production by inhibiting (+)-geodin biosynthesis in Aspergillus terreus. New Biotechnology, 52(April), 19–24. https://doi.org/10.1016/j.nbt.2019.04.003.
Boynton, Z. L., Bennett, G. N., & Rudolph, F. B. (1994). Intracellular concentrations of coenzyme A and its derivatives from Clostridium acetobutylicum ATCC 824 and their roles in enzyme regulation. Applied and Environmental Microbiology, 60(1), 39–44. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=201266&tool=pmcentrez&rendertype=abstract.
Hu, Y., Zhu, Z., Nielsen, J., & Siewers, V. (2019). Engineering Saccharomyces cerevisiae cells for production of fatty acid-derived biofuels and chemicals. Open Biology, 9(5), 190049. https://doi.org/10.1098/rsob.190049.
Ullah, N., Shahzad, K., & Wang, M. (2021). The role of metabolic engineering technologies for the production of fatty acids in yeast. Biology, 10(7), 632. https://doi.org/10.3390/biology10070632.
Krivoruchko, A., Zhang, Y., Siewers, V., Chen, Y., & Nielsen, J. (2014). Microbial acetyl-CoA metabolism and metabolic engineering. Metabolic Engineering, 28, 28–42. https://doi.org/10.1016/j.ymben.2014.11.009.
Milke, L., & Marienhagen, J. (2020). Engineering intracellular malonyl-CoA availability in microbial hosts and its impact on polyketide and fatty acid synthesis. Applied Microbiology and Biotechnology, 104(14), 6057–6065. https://doi.org/10.1007/s00253-020-10643-7.
Korman, T. P., Ames, B., & Sheryl Tsai, S.-C. (2010). Structural enzymology of polyketide synthase: The structure–sequence–function correlation. In Comprehensive natural products II (pp. 305–345). Elsevier. https://doi.org/10.1016/B978-008045382-8.00020-4.
Möller, E. M., Bahnweg, G., Sandermann, H., & Geiger, H. H. (1992). A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Research, 20(22), 6115–6116. https://doi.org/10.1093/nar/20.22.6115.
Komatsu, M., Uchiyama, T., Omura, S., Cane, D. E., & Ikeda, H. (2010). Genome-minimized Streptomyces host for the heterologous expression of secondary metabolism. Proceedings of the National Academy of Sciences of the United States of America, 107(6), 2646–2651. https://doi.org/10.1073/pnas.0914833107.
Martins-Santana, L., Nora, L. C., Sanches-Medeiros, A., Lovate, G. L., Murilo, M. H., & Silva-Rocha, R. (2018). Systems and synthetic biology approaches to engineer fungi for fine chemical production. Frontiers in Bioengineering and Biotechnology, 6(OCT), 1–18. https://doi.org/10.3389/fbioe.2018.00117.
Fang, X., & Qu, Y. (2018). Fungal cellulolytic enzymes: Microbial production and application (pp. 1–282). Springer. https://doi.org/10.1007/978-981-13-0749-2.
Li, J., Zhang, Y., Li, J., Sun, T., & Tian, C. (2020). Metabolic engineering of the cellulolytic thermophilic fungus Myceliophthora thermophila to produce ethanol from cellobiose. Biotechnology for Biofuels, 13(1), 1–15. https://doi.org/10.1186/s13068-020-1661-y.
Tong, Z., Zheng, X., Tong, Y., Shi, Y. C., & Sun, J. (2019). Systems metabolic engineering for citric acid production by Aspergillus niger in the post-genomic era. Microbial Cell Factories, 18(1), 1–15. https://doi.org/10.1186/s12934-019-1064-6.
Wang, Q., Zhong, C., & Xiao, H. (2020). Genetic engineering of filamentous fungi for efficient protein expression and secretion. Frontiers in Bioengineering and Biotechnology, 8(March), 1–8. https://doi.org/10.3389/fbioe.2020.00293.
Mojzita, D., Wiebe, M., Hilditch, S., Boer, H., Penttilä, M., & Richard, P. (2010). Metabolic engineering of fungal strains for conversion of d-galacturonate to meso-galactarate. Applied and Environmental Microbiology, 76(1), 169–175. https://doi.org/10.1128/AEM.02273-09.
Feng, X., Lian, J., & Zhao, H. (2015). Metabolic engineering of Saccharomyces cerevisiae to improve 1-hexadecanol production. Metabolic Engineering, 27, 10–19. https://doi.org/10.1016/j.ymben.2014.10.001.
Pickens, L., Tang, Y., & Chooi, Y. H. (2011). Metabolic engineering for the production of natural products. Annual Review of Chemical and Biomolecular Engineering, 23(1), 211–236. https://doi.org/10.1146/061010-114209.
Zhang, S., Yang, W., Chen, H., Liu, B., Lin, B., & Tao, Y. (2019). Metabolic engineering for efficient supply of acetyl-CoA from different carbon sources in Escherichia coli. Microbial Cell Factories, 18(1), 1–11. https://doi.org/10.1186/s12934-019-1177-y.
Srivastava, R. K., Akhtar, N., Verma, M., & Imandi, S. B. (2020). Primary metabolites from overproducing microbial system using sustainable substrates. Biotechnology and Applied Biochemistry, 67(6), 852–874. https://doi.org/10.1002/bab.1927.
Casas López, J. L., Rodríguez Porcel, E. M., Vilches Ferrón, M. A., Sánchez Pérez, J. A., Fernández Sevilla, J. M., & Chisti, Y. (2004). Lovastatin inhibits its own synthesis in Aspergillus terreus. Journal of Industrial Microbiology and Biotechnology, 31(1), 48–50. https://doi.org/10.1007/s10295-004-0108-y.
Jia, Z., Zhang, X., Cao, X., Liu, J., & Qin, B. (2011). Production of lovastatin by a self-resistant mutant of Aspergillus terreus. Annals of Microbiology, 61(3), 615–621. https://doi.org/10.1007/s13213-010-0180-7.
Podkowiński, J., & Tworak, A. (2011). Acetyl-coenzyme A carboxylase—An attractive enzyme for biotechnology. Biotechnologia, 92(4), 321–335.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hasan, H., Abd Rahim, M.H., Campbell, L. et al. Increasing Lovastatin Production by Re-routing the Precursors Flow of Aspergillus terreus via Metabolic Engineering. Mol Biotechnol 64, 90–99 (2022). https://doi.org/10.1007/s12033-021-00393-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-021-00393-w