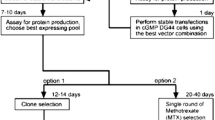
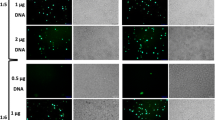
Abstract
Monoclonal antibodies (mAbs) are one of the most significant molecules in protein therapeutics. They are employed in the field of immunology, oncology and organ transplant. They have been also been employed for alleviating several bacterial and viral infections. Moreover, they have revolutionized the area of targeted therapy and improved the quality of treatments, as compared to other cytotoxic drugs and therapies. mAbs bind to specific molecules on the antigen and exhibit specificity towards that molecule, i.e. epitope. Thus, mAbs have immense opportunity to be explored for personalized therapy. The introduction of targeted mAb-based therapeutics has promoted many important scientific achievements in rheumatology. This has warranted additional investigations for developing newer mAb producing clones, to supplement the limited industrial production of certain mAb therapeutics. In this investigation, an integrative approach comprising optimized expression, selection and expansion was adopted to develop a mammalian cell line expressing mAb against TNF-α.The resulting stable clone is anticipated to serve as an economic alternative to the industrial clones, especially for research purposes. The clone was constructed for development of biosimilar of the highly valued therapeutic antibody, Humira.











Similar content being viewed by others
Abbreviations
- mAb:
-
Monoclonal antibody
- TNF- α:
-
Tumor necrosis factor alpha
- CHO-K1:
-
Chinese hamster ovary –K1
- G418:
-
Geneticin
- FBS:
-
Fetal bovine serum
- BSA:
-
Bovine serum albumin
- ADCC:
-
Antibody dependent cell mediated cytoxicity
- HRP:
-
Horseradish peroxidase
- ELISA:
-
Enzyme-linked immunosorbent assay
- HT:
-
Hypoxanthine
- TMB:
-
Tetramethylenebenzidine
- PCD:
-
Picogram protein/cell/day
- HC:
-
Heavy chain
- LC:
-
Light chain
- IRES:
-
Internal ribosome entry site
- FITC:
-
Fluorescein isothiocyanate
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- IgG:
-
Immunoglobulin
- Fc:
-
Fragment crystallizable region
References
Gronemeyer, P., Ditz, R., & Strube, J. (2014). Trends in upstream and downstream process development for antibody manufacturing. Bioengineering, 1(4), 188–212
Gupta, K., et al. (2019). Vector-related stratagems for enhanced monoclonal antibody production in mammalian cells. Biotechnology Advances
Chames, P., et al. (2009). Therapeutic antibodies: Successes, limitations and hopes for the future. British Journal of Pharmacology, 157(2), 220–233
Voronina, E., et al. (2016). Design of a stable cell line producing a recombinant monoclonal anti-TNFα antibody based on a CHO cell line. Springerplus, 5(1), 1584
Alonso-Ruiz, A., et al. (2008). Tumor necrosis factor alpha drugs in rheumatoid arthritis: Systematic review and metaanalysis of efficacy and safety. BMC Musculoskeletal Disorders, 9(1), 52
Kunert, R., & Reinhart, D. (2016). Advances in recombinant antibody manufacturing. Applied Microbiology and Biotechnology, 100(8), 3451–3461
Azevedo, V. F., et al. (2016). Adalimumab: A review of the reference product and biosimilars. Biosimilars, 6, 29
Furst, D., et al. (2007). (2007) Updated consensus statement on biological agents for the treatment of rheumatic diseases. Annals of the Rheumatic Diseases, 66(3), iii 2-iii22
Scott, D., & Kingsley, G. (2006). Tumor necrosis factor inhibitors for rheumatoid arthritis. New England Journal of Medicine, 355(7), 704–712
Li, F., Shen, A., & Amanullah, A. (2010). Cell culture processes in monoclonal antibody production. (pp. 1–38). Pharmaceutical Sciences Encyclopedia: Drug Discovery, Development, and Manufacturing.
Costa, A. R., et al. (2010). Guidelines to cell engineering for monoclonal antibody production. European Journal of Pharmaceutics and Biopharmaceutics, 74(2), 127–138
Xu, X., et al. (2011). The genomic sequence of the Chinese hamster ovary (CHO)-K1 cell line. Nature biotechnology, 29(8), 735
Wurm, F. M. (2013). CHO quasispecies—Implications for manufacturing processes. Processes, 1(3), 296–311
Sinici, I., et al. (2006). Comparison of HCMV IE and EF-1 promoters for the stable expression of β-subunit of hexosaminidase in CHO cell lines. Biochemical Genetics, 44(3–4), 168–175
Kober, L., Zehe, C., & Bode, J. (2013). Optimized signal peptides for the development of high expressing CHO cell lines. Biotechnology and Bioengineering, 110(4), 1164–1173
Sandbichler, A. M., Aschberger, T., & Pelster, B. (2013). A method to evaluate the efficiency of transfection reagents in an adherent zebrafish cell line. BioResearch Open Access, 2(1), 20–27
Harlow, E., & Lane, D. (1999). Using Antibodies Cold Spring Harbor Lab Press. Cold Spring Harbor Press.
Lipman, N. S., et al. (2005). Monoclonal versus polyclonal antibodies: Distinguishing characteristics, applications, and information resources. ILAR Journal, 46(3), 258–268
Gross, A., et al. (2015). Technologies for single-cell isolation. International Journal of Molecular Sciences, 16(8), 16897–16919
Ozturk, S. S., & Palsson, B. O. (1991). Physiological changes during the adaptation of hybridoma cells to low serum and serum-free media. Biotechnology and Bioengineering, 37(1), 35–46
Rodrigues, M. E., et al. (2013). Advances and drawbacks of the adaptation to serum-free culture of CHO-K1 cells for monoclonal antibody production. Applied Biochemistry and Biotechnology, 169(4), 1279–1291
Reinhart, D., et al. (2015). Benchmarking of commercially available CHO cell culture media for antibody production. Applied Microbiology and Biotechnology, 99(11), 4645–4657
Pan, X., et al. (2017). Selection of chemically defined media for CHO cell fed-batch culture processes. Cytotechnology, 69(1), 39–56
Lee, J. J., et al. (2019). Demonstration of functional similarity of a biosimilar adalimumab SB5 to Humira®. Biologicals, 58, 7–15
Acknowledgements
Kritika Gupta is thankful to Indian Council of Medical Research(ICMR), No. 3/1/3/JRF-2015/HRD-LS/29/31232/85 for fellowship and financial support. Deepak Modi is thankful to ICMR for financial support. Prajakta Dandekar is thankful to Department of Science and Technology (DST), Science and Engineering Research Board (SERB): Ramanujan Fellowship Grant (SR/S2/RJN-139/2011) and UGC for Start-up Grant20-1/2012 (BSR)/20-1(10)/2012(BSR). Ratnesh Jain is thankful to DBT, Ministry of Science and Technology: Ramalingaswami Fellowship Grant (BT/RLF/Re-entry/51/2011).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All the contributing authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gupta, K., Modi, D., Jain, R. et al. A Stable CHO K1 Cell Line for Producing Recombinant Monoclonal Antibody Against TNF-α. Mol Biotechnol 63, 828–839 (2021). https://doi.org/10.1007/s12033-021-00329-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-021-00329-4