Abstract
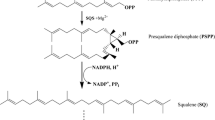
Squalene hopene cyclases catalyse the conversion of a linear substrate squalene to a cyclic product with high stereo-selectivity.The enzyme squalene hopene cyclase from Pseudomonas mendocina expressed in E. coli BL21 (DE3) was evaluated for its synthetic drug transforming ability. Nine synthetic drugs were selected as substrates for biotransformation reactions by the enzyme. The homology modelling of the protein and docking of the selected ligands were performed using GOLD suite docking software. The drug which showed maximum binding with the active-site residues of the enzyme was selected for biotransformation studies. On transformation with the enzyme, Glibenclamide, the selected antidiabetic drug alone showed significant changes in the FT/IR spectra; hence, it was selected for LCMS analysis to confirm the transformations. From the chromatogram and MS spectra, the mono-oxygenation of the product due to the enzymatic activity was confirmed. The drug transforming ability of the purified SHC could be used as an ideal tool for the generation of new and active substrate derivatives.




Similar content being viewed by others
References
Sultana, N., & Saify, Z. S. (2013). Enzymatic biotransformation of terpenes as bioactive agents. Journal of Enzyme Inhibition and Medicinal Chemistry,28, 1113–1128. https://doi.org/10.3109/14756366.2012.727411.
Smitha, M. S., Singh, S., & Singh, R. (2017). Microbial biotransformation: A process for chemical alterations. Journal of Bacteriology & Mycology Open Access,4(2), 85. https://doi.org/10.15406/jbmoa.2017.04.00085.
Bicas, J. L., Fontanille, P., Pastore, G. M., & Larroche, C. (2008). Characterization of monoterpene biotransformation in two pseudomonads. Journal of Applied Microbiology,105, 1991–2001. https://doi.org/10.1111/j.1365-2672.2008.03923.x.
Schrittwieser, J. H., Velikogne, S., Hall, M., & Kroutil, W. (2018). Artificial biocatalytic linear cascades for preparation of organic molecules. Chemical Reviews,118, 270–348. https://doi.org/10.1021/acs.chemrev.7b00033.
Germaine, K., Keogh, E., Garcia-Cabellos, G., Borremans, B., Van Der Lelie, D., Barac, T., et al. (2004). Colonisation of poplar trees by gfp expressing bacterial endophytes. FEMS Microbiology Ecology,48, 109–118. https://doi.org/10.1016/j.femsec.2003.12.009.
Wagner, M. R., Lundberg, D. S., Del Rio, T. G., Tringe, S. G., Dangl, J. L., & Mitchell-Olds, T. (2016). Host genotype and age shape the leaf and root microbiomes of a wild perennial plant. Nature Communications. https://doi.org/10.1038/ncomms12151.
Abe, I. (2007). Enzymatic synthesis of cyclic triterpenes. Natural Products Reports,24, 1311. https://doi.org/10.1039/b616857b.
Schauer, F., & Borriss, R. (2004). Biocatalysis and biotransformation. In J. S. Tkacz & L. Lange (Eds.), Advances in fungal biotechnology for industry, agriculture, and medicine (pp. 237–306). Boston, MA: Springer.
Nair, I. M., & Jayachandran, K. (2017). A novel strain of Pantoea eucrina endophyte of Murraya koenigii with squalene cyclase activity. LIFE: International Journal of Health and Life-Sciences,3, 161–177. https://doi.org/10.20319/lijhls.2017.32.161177.
Nair, I. M., & Kochupurakal, J. (2019). In silico characterization and over-expression of squalene hopene cyclase from Pseudomonas mendocina. 3 Biotech,9, 1–7. https://doi.org/10.1007/s13205-019-1901-7.
Longo, M. A., & Sanromán, M. A. (2006). Production of food aroma compounds: Microbial and enzymatic methodologies. Food Technology and Biotechnology,44, 335–353.
Ravindran, S., Basu, S., Gorti, S. K. K., Surve, P., & Sloka, N. (2013). Metabolic profile of glyburide in human liver microsomes using LC-DAD-Q-TRAP-MS/MS. Biomedical Chromatography.,27, 575–582. https://doi.org/10.1002/bmc.2830.
Wendt, K. U., Poralla, K., & Schulz, G. E. (1997). Structure and function of a squalene cyclase. Science,277, 1811–1815.
Tanaka, H., Noma, H., Noguchi, H., & Abe, I. (2006). Enzymatic formation of pyrrole-containing novel cyclic polyprenoids by bacterial squalene:hopene cyclase. Tetrahedron Letters,47, 3085–3089. https://doi.org/10.1016/j.tetlet.2006.02.151.
Bansal, G., Singh, M., Jindal, K., & Singh, S. (2008). Ultraviolet-photodiode array and high-performance liquid chromatographic/mass spectrometric studies on forced degradation behavior of glibenclamide and development of a validated stability-indicating method. Journal of AOAC International,91, 709.
Hussain, R., Ahmed, M., Khan, T. A., & Akhter, Y. (2020). Fungal P450 monooxygenases—The diversity in catalysis and their promising roles in biocontrol activity. Applied Microbiology and Biotechnology,104, 989–999. https://doi.org/10.1007/s00253-019-10305-3.
Arfeen, M., Patel, D. S., Abbat, S., Taxak, N., & Bharatam, P. V. (2014). Importance of cytochromes in cyclization reactions: Quantum chemical study on a model reaction of proguanil to cycloguanil. Journal of Computational Chemistry,35, 2047–2055. https://doi.org/10.1002/jcc.23719.
Wei, Y., Lu, C., Jiang, S., Zhang, Y., Li, Q., Bai, W.-J., et al. (2020). Directed evolution of a tryptophan 2,3-dioxygenase for diastereoselective monooxygenation of tryptophans. Angewandte Chemie International Edition. https://doi.org/10.1002/anie.201911825.
Acknowledgements
We are thankful to Dr.Amjesh Ambu, Junior Scientist Department of Computational Biology and Bioinformatics, University of Kerala for the docking studies of the enzyme and SIIC, University of Kerala for the LCMS analysis
Author information
Authors and Affiliations
Contributions
The author IMN has contributed to the execution of the work and the preparation of the manuscript. The corresponding author KJ contributed to the planning of the work and correction of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nair, I.M., Jayachandran, K. In Vitro Enzymatic Conversion of Glibenclamide Using Squalene Hopene Cyclase from Pseudomonas mendocina Expressed in E. coli BL21 (DE3). Mol Biotechnol 62, 456–465 (2020). https://doi.org/10.1007/s12033-020-00264-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-020-00264-w