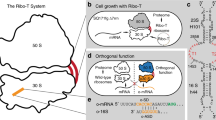
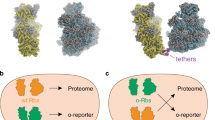
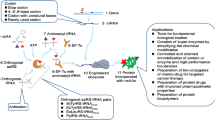
Abstract
The ribosome is an essential organelle in charge of the translational processes in all kinds of cells. Currently, the scenario of its function has been significantly expanded from the classic machine for protein synthesis to a regulatory platform for quality control to maintain the protein homeostasis in a living cell. The ribosome is much more than a mechanical device with a static structure: it is inherently dynamic in structure and function, especially in response to the environmental fluctuations. Considerable effort has been made to regulate its structure and physiological function by engineering the components of a ribosome. The findings of the pioneering studies significantly deepened our understanding of a ribosome and exemplified how a ribosome could be engineered for biotechnology purposes in the era of synthetic biology. The engineering of ribosome offered highly accessible methods capable of comprehensively optimizing the performance of strains of industrial importance. In this article, the relevant recent advances were systematically reviewed.



Similar content being viewed by others
References
Bozhuyuk, K. A. J., Fleischhacker, F., Linck, A., Wesche, F., Tietze, A., Niesert, C. P., et al. (2018). De novo design and engineering of non-ribosomal peptide synthetases. Nature Chemistry,10, 275–281.
Palade, G. E. (1955). A small particulate component of the cytoplasm. Journal of Biophysical and Biochemical Cytology,1, 59–68.
Capel, M. S., Engelman, D. M., Freeborn, B. R., Kjeldgaard, M., Langer, J. A., Ramakrishnan, V., et al. (1987). A complete mapping of the proteins in the small ribosomal subunit of Escherichia coli. Science,238, 1403–1406.
Ban, N., Nissen, P., Hansen, J., Moore, P. B., & Steitz, T. A. (2000). The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science,289, 905.
Bashan, A., Agmon, I., Zarivach, R., Schluenzen, F., Harms, J., Berisio, R., et al. (2003). Structural basis of the ribosomal machinery for peptide bond formation, translocation, and nascent chain progression. Molecular Cell,11, 91–102.
Wada, A. (1998). Growth phase coupled modulation of Escherichia coli ribosomes. Genes to Cells,3, 203–208.
Wada, A., Mikkola, R., Kurland, C. G., & Ishihama, A. (2000). Growth phase-coupled changes of the ribosome profile in natural isolates and laboratory strains of Escherichia coli. Journal of Bacteriology,182, 2893–2899.
Slavov, N., Semrau, S., Airoldi, E., Budnik, B., & van Oudenaarden, A. (2015). Differential stoichiometry among core ribosomal proteins. Cell Reports,13, 865–873.
Kondrashov, N., Pusic, A., Stumpf, C. R., Shimizu, K., Hsieh, A. C., Ishijima, J., et al. (2011). Ribosome-mediated specificity in Hox mRNA translation and vertebrate tissue patterning. Cell,145, 383–397.
Degenhardt, R. F., & Bonham-Smith, P. C. (2008). Arabidopsis ribosomal proteins RPL23aA and RPL23aB are differentially targeted to the nucleolus and are disparately required for normal development. Plant Physiology,147, 128–142.
Kim, K. Y., Park, S. W., Chung, Y. S., Chung, C. H., Kim, J. I., & Lee, J. H. (2004). Molecular cloning of low-temperature-inducible ribosomal proteins from soybean. Journal of Experimental Botany,55, 1153–1155.
Simsek, D., & Barna, M. (2017). An emerging role for the ribosome as a nexus for post-translational modifications. Current Opinion in Cell Biology,45, 92–101.
Nesterchuk, M. V., Sergiev, P. V., & Dontsova, O. A. (2011). Posttranslational modifications of ribosomal proteins in Escherichia coli. Acta Naturae,3, 22–33.
Genuth, N. R., & Barna, M. (2018). The discovery of ribosome heterogeneity and its implications for gene regulation and organismal life. Molecular Cell,71, 364–374.
Xue, S., & Barna, M. (2012). Specialized ribosomes: a new frontier in gene regulation and organismal biology. Nature Reviews Molecular Cell Biology,13, 355–369.
Orelle, C., Carlson, E. D., Szal, T., Florin, T., Jewett, M. C., & Mankin, A. S. (2015). Protein synthesis by ribosomes with tethered subunits. Nature,524, 119–124.
Kannan, K., Tsvetanova, B., Chuang, R. Y., Noskov, V. N., Assad-Garcia, N., Ma, L., et al. (2016). One step engineering of the small-subunit ribosomal RNA using CRISPR/Cas9. Science Reports,6, 30714.
Vesper, O., Amitai, S., Belitsky, M., Byrgazov, K., Kaberdina, A. C., Engelberg-Kulka, H., et al. (2011). Selective translation of leaderless mRNAs by specialized ribosomes generated by MazF in Escherichia coli. Cell,147, 147–157.
Luidalepp, H., Berger, S., Joss, O., Tenson, T., & Polacek, N. (2016). Ribosome shut-down by 16S rRNA fragmentation in stationary-phase Escherichia coli. Journal of Molecular Biology,428, 2237–2247.
Oron-Gottesman, A., Sauert, M., Moll, I., & Engelberg-Kulka, H. (2016). A Stress-induced bias in the reading of the genetic code in Escherichia coli. MBio,7, e01855.
Nikolic, N., Didara, Z., & Moll, I. (2017). MazF activation promotes translational heterogeneity of the grcA mRNA in Escherichia coli populations. PeerJ,5, e3830.
Nikolic, N., Bergmiller, T., Vandervelde, A., Albanese, T. G., Gelens, L., & Moll, I. (2018). Autoregulation of mazEF expression underlies growth heterogeneity in bacterial populations. Nucleic Acids Research,46, 2918–2931.
Mets, T., Kasvandik, S., Saarma, M., Maivali, U., Tenson, T., & Kaldalu, N. (2019). Fragmentation of Escherichia coli mRNA by MazF and MqsR. Biochimie,156, 79–91.
Kohanski, M. A., Dwyer, D. J., & Collins, J. J. (2010). How antibiotics kill bacteria: from targets to networks. Nature Reviews Microbiology,8, 423–435.
Funatsu, G., & Wittmann, H. G. (1972). Ribosomal proteins. 33. Location of amino-acid replacements in protein S12 isolated from Escherichia coli mutants resistant to streptomycin. Journal of Molecular Biology,68, 547–550.
Finken, M., Kirschner, P., Meier, A., Wrede, A., & Bottger, E. C. (1993). Molecular basis of streptomycin resistance in Mycobacterium tuberculosis: alterations of the ribosomal protein S12 gene and point mutations within a functional 16S ribosomal RNA pseudoknot. Molecular Microbiology,9, 1239–1246.
Honore, N., & Cole, S. T. (1994). Streptomycin resistance in mycobacteria. Antimicrobial Agents and Chemotherapy,38, 238–242.
Meier, A., Kirschner, P., Bange, F. C., Vogel, U., & Bottger, E. C. (1994). Genetic alterations in streptomycin-resistant Mycobacterium tuberculosis: mapping of mutations conferring resistance. Antimicrobial Agents and Chemotherapy,38, 228–233.
Shima, J., Hesketh, A., Okamoto, S., Kawamoto, S., & Ochi, K. (1996). Induction of actinorhodin production by rpsL (encoding ribosomal protein S12) mutations that confer streptomycin resistance in Streptomyces lividans and Streptomyces coelicolor A3(2). Journal of Bacteriology,178, 7276–7284.
Suzuki, T., Seta, K., Nishikawa, C., Hara, E., Shigeno, T., & Nakajima-Kambe, T. (2015). Improved ethanol tolerance and ethanol production from glycerol in a streptomycin-resistant Klebsiella variicola mutant obtained by ribosome engineering. Bioresource Technology,176, 156–162.
Chen, L., Shang, G., Yuan, W., Wu, Y., & Bai, F. (2012). Screening of Clostridium strains through ribosome engineering for improved butanol production. Sheng Wu Gong Cheng Xue Bao,28, 1048–1058.
Ochi, K., Okamoto, S., Tozawa, Y., Inaoka, T., Hosaka, T., Xu, J., et al. (2004). Ribosome engineering and secondary metabolite production. Advances in Applied Microbiology,56, 155–184.
Zhu, S., Duan, Y., & Huang, Y. (2019). The application of ribosome engineering to natural product discovery and yield improvement in Streptomyces. Antibiotics (Basel). https://doi.org/10.3390/antibiotics8030133.
Wang, L., Chen, X., Wu, G., Li, S., Zeng, X., Ren, X., et al. (2017). Enhanced epsilon-poly-L-lysine production by inducing double antibiotic-resistant mutations in Streptomyces albulus. Bioprocess and Biosystems Engineering,40, 271–283.
Tanaka, Y., Kasahara, K., Izawa, M., & Ochi, K. (2017). Applicability of ribosome engineering to vitamin B12 production by Propionibacterium shermanii. Bioscience, Biotechnology, and Biochemistry,81, 1636–1641.
Kurosawa, K., Hosaka, T., Tamehiro, N., Inaoka, T., & Ochi, K. (2006). Improvement of alpha-amylase production by modulation of ribosomal component protein S12 in Bacillus subtilis 168. Applied and Environment Microbiology,72, 71–77.
Xu, J., Tozawa, Y., Lai, C., Hayashi, H., & Ochi, K. (2002). A rifampicin resistance mutation in the rpoB gene confers ppGpp-independent antibiotic production in Streptomyces coelicolor A3(2). Molecular Genetics and Genomics,268, 179–189.
Halfon, Y., Jimenez-Fernandez, A., La Rosa, R., Espinosa Portero, R., Krogh Johansen, H., Matzov, D., et al. (2019). Structure of Pseudomonas aeruginosa ribosomes from an aminoglycoside-resistant clinical isolate. Proceedings of the National Academy of Sciences of the United States of America,116, 22275–22281.
Zhao, Y., Song, Z., Ma, Z., Bechthold, A., & Yu, X. (2019). Sequential improvement of rimocidin production in Streptomyces rimosus M527 by introduction of cumulative drug-resistance mutations. Journal of Industrial Microbiology and Biotechnology,46, 697–708.
Tamehiro, N., Hosaka, T., Xu, J., Hu, H., Otake, N., & Ochi, K. (2003). Innovative approach for improvement of an antibiotic-overproducing industrial strain of Streptomyces albus. Applied and Environment Microbiology,69, 6412–6417.
Okamoto-Hosoya, Y., Hosaka, T., & Ochi, K. (2003). An aberrant protein synthesis activity is linked with antibiotic overproduction in rpsL mutants of Streptomyces coelicolor A3(2). Microbiology,149, 3299–3309.
Hosaka, T., Xu, J., & Ochi, K. (2006). Increased expression of ribosome recycling factor is responsible for the enhanced protein synthesis during the late growth phase in an antibiotic-overproducing Streptomyces coelicolor ribosomal rpsL mutant. Molecular Microbiology,61, 883–897.
Lopatniuk, M., Myronovskyi, M., Nottebrock, A., Busche, T., Kalinowski, J., Ostash, B., et al. (2019). Effect of "ribosome engineering" on the transcription level and production of S. albus indigenous secondary metabolites. Applied Microbiology and Biotechnology,103, 7097–7110.
Zhang, K., Mohsin, A., Dai, Y., Chen, Z., Zhuang, Y., Chu, J., et al. (2019). Combinatorial effect of ARTP mutagenesis and ribosome engineering on an industrial strain of Streptomyces albus S12 for enhanced biosynthesis of Salinomycin. Frontiers in Bioengineering and Biotechnology,7, 212.
Noren, C. J., Anthony-Cahill, S. J., Griffith, M. C., & Schultz, P. G. (1989). A general method for site-specific incorporation of unnatural amino acids into proteins. Science,244, 182–188.
Chin, J. W. (2017). Expanding and reprogramming the genetic code. Nature,550, 53–60.
Smolskaya, S., & Andreev, Y. A. (2019). Site-specific incorporation of unnatural amino acids into escherichia coli recombinant protein: methodology development and recent achievement. Biomolecules. https://doi.org/10.1111/eip.12846.
Chin, J. W. (2014). Expanding and reprogramming the genetic code of cells and animals. Annual Review of Biochemistry,83, 379–408.
Wang, L., Brock, A., Herberich, B., & Schultz, P. G. (2001). Expanding the genetic code of Escherichia coli. Science,292, 498–500.
Gao, W., Cho, E., Liu, Y., & Lu, Y. (2019). Advances and challenges in cell-free incorporation of unnatural amino acids into proteins. Frontiers in Pharmacology,10, 611.
Hoshika, S., Leal, N. A., Kim, M. J., Kim, M. S., Karalkar, N. B., Kim, H. J., et al. (2019). Hachimoji DNA and RNA: a genetic system with eight building blocks. Science,363, 884–887.
Magliery, T. J., Anderson, J. C., & Schultz, P. G. (2001). Expanding the genetic code: Selection of efficient suppressors of four-base codons and identification of "shifty" four-base codons with a library approach in Escherichia coli. Journal of Molecular Biology,307, 755–769.
Suddala, K. C., & Zhang, J. (2019). High-affinity recognition of specific tRNAs by an mRNA anticodon-binding groove. Nature Structural & Molecular Biology,26, 1114–1122.
Battaglia, R. A., Grigg, J. C., & Ke, A. (2019). Structural basis for tRNA decoding and aminoacylation sensing by T-box riboregulators. Nature Structural & Molecular Biology,26, 1106–1113.
Li, S., Su, Z., Lehmann, J., Stamatopoulou, V., Giarimoglou, N., Henderson, F. E., et al. (2019). Structural basis of amino acid surveillance by higher-order tRNA-mRNA interactions. Nature Structural & Molecular Biology,26, 1094–1105.
d'Aquino, A. E., Kim, D. S., & Jewett, M. C. (2018). Engineered ribosomes for basic science and synthetic biology. Annual Review of Chemical and Biomolecular Engineering,9, 311–340.
Davis, L., & Chin, J. W. (2012). Designer proteins: applications of genetic code expansion in cell biology. Nature Reviews Molecular Cell Biology,13, 168–182.
Lee, J., Schwieter, K. E., Watkins, A. M., Kim, D. S., Yu, H., Schwarz, K. J., et al. (2019). Expanding the limits of the second genetic code with ribozymes. Nature Communications,10, 5097.
Gan, R., Perez, J. G., Carlson, E. D., Ntai, I., Isaacs, F. J., Kelleher, N. L., et al. (2017). Translation system engineering in Escherichia coli enhances non-canonical amino acid incorporation into proteins. Biotechnology and Bioengineering,114, 1074–1086.
Isaacs, F. J., Carr, P. A., Wang, H. H., Lajoie, M. J., Sterling, B., Kraal, L., et al. (2011). Precise manipulation of chromosomes in vivo enables genome-wide codon replacement. Science,333, 348–353.
Cui, Z., Mureev, S., Polinkovsky, M. E., Tnimov, Z., Guo, Z., Durek, T., et al. (2017). Combining sense and nonsense codon reassignment for site-selective protein modification with unnatural amino acids. ACS Synthetic Biology,6, 535–544.
Wang, L., & Schultz, P. G. (2004). Expanding the genetic code. Angewandte Chemie (International ed. in English),44, 34–66.
Cirino, P. C., Tang, Y., Takahashi, K., Tirrell, D. A., & Arnold, F. H. (2003). Global incorporation of norleucine in place of methionine in cytochrome P450 BM-3 heme domain increases peroxygenase activity. Biotechnology and Bioengineering,83, 729–734.
Addy, P. S., Erickson, S. B., Italia, J. S., & Chatterjee, A. (2019). Labeling proteins at site-specifically incorporated 5-hydroxytryptophan residues using a chemoselective rapid azo-coupling reaction. Methods in Molecular Biology,2033, 239–251.
Merten, H., Schaefer, J. V., Brandl, F., Zangemeister-Wittke, U., & Pluckthun, A. (2019). Facile site-specific multiconjugation strategies in recombinant proteins produced in Bacteria. Methods in Molecular Biology,2033, 253–273.
Spicer, C. D., & Davis, B. G. (2014). Selective chemical protein modification. Nature Communications,5, 4740.
Seo, M. H., Han, J., Jin, Z., Lee, D. W., Park, H. S., & Kim, H. S. (2011). Controlled and oriented immobilization of protein by site-specific incorporation of unnatural amino acid. Analytical Chemistry,83, 2841–2845.
Raliski, B. K., Howard, C. A., & Young, D. D. (2014). Site-specific protein immobilization using unnatural amino acids. Bioconjugate Chemistry,25, 1916–1920.
Wu, J. C., Hutchings, C. H., Lindsay, M. J., Werner, C. J., & Bundy, B. C. (2015). Enhanced enzyme stability through site-directed covalent immobilization. Journal of Biotechnology,193, 83–90.
Bednar, R. M., Golbek, T. W., Kean, K. M., Brown, W. J., Jana, S., Baio, J. E., et al. (2019). Immobilization of proteins with controlled load and orientation. ACS Applied Materials & Interfaces,11, 36391–36398.
Friedman, M., Ozer, E., Kushmaro, A., & Alfonta, L. (2019). Cellular localization of cytochrome bd in cyanobacteria using genetic code expansion. Biotechnology and Bioengineering,117, 523–530.
Albayrak, C., & Swartz, J. R. (2014). Direct polymerization of proteins. ACS Synthetic Biology,3, 353–362.
Eiselt, E., Gonzalez, S., Martin, C., Chartier, M., Betti, C., Longpre, J. M., et al. (2019). Neurotensin analogues containing cyclic surrogates of tyrosine at position 11 improve NTS2 selectivity leading to analgesia without Hypotension and hypothermia. ACS Chemical Neuroscience,10, 4535–4544.
Kelemen, R. E., Mukherjee, R., Cao, X., Erickson, S. B., Zheng, Y., & Chatterjee, A. (2016). A precise chemical strategy to alter the receptor specificity of the adeno-associated virus. Angewandte Chemie (International ed. in English),55, 10645–10649.
Huang, Y., & Liu, T. (2018). Therapeutic applications of genetic code expansion. Synthetic and Systems Biotechnology,3, 150–158.
Zhang, Z. W., Gildersleeve, J., Yang, Y. Y., Xu, R., Loo, J. A., Uryu, S., et al. (2004). A new strategy for the synthesis of glycoproteins. Science,303, 371–373.
Acknowledgement
This publication was financially supported by the Nanhu Scholars Program for Young Scholars of XYNU, Startup Program, and Key Program for Natural Science Exploration of XYNU (2017-ZDYY-160), and Natural Science Foundation of China (U180410).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, T., Liang, C., An, Y. et al. Engineering the Translational Machinery for Biotechnology Applications. Mol Biotechnol 62, 219–227 (2020). https://doi.org/10.1007/s12033-020-00246-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-020-00246-y