Abstract
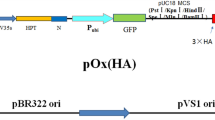
Tall fescue plants are widely exposed to white grubs. Regarding the rate of damage caused by the white grubs to tall fescue and difficulty of its ecological and economical control, production of resistant cultivars is a priority. In this experiment, for the first time, we report production of transgenic lines resistant to white grub using CR8DB gene. For this, mature seeds were placed on MS basal medium with 0–15 mg L−1 2,4-D for callogenesis and 0–1.75 mg L−1 BA for regeneration. ‘Asterix’ (54.11%) in 7.5 and ‘Talladega’ (52.53%) in 10 mg L−1 2,4-D showed maximum callogenesis. Regeneration percentage was higher in 0.5 mg L−1 BA. Agrobacterium tumefaciens strain LBA4404 harbouring binary vector pCAMBIA 1301 with CRY8DB gene, which contains HPTII gene and uidA and various types MS media were used for transformation of calli. The highest percentage of gus enzyme activity and hygromycin resistance in calli was related to the modified medium type 11. The PCR and RT-PCR analysis was done to confirm the presence and expression of the target gene in transgenic 5 lines in ‘Asterix’ and 3 lines in ‘Talladega’. According to bioassay, larvae mortality of 91.66% was observed in transgenic plants, whereas it was 15.52% in control plants.











Similar content being viewed by others
References
Lilly, P. J., Jenkins, J. C., & Carroll, M. J. (2015). Management alters C allocation in turfgrass lawns. Landscape and Urban Planning Journal, 134, 119–126. https://doi.org/10.1016/j.landurbplan.2014.10.011.
Saeedi Pooya, E., Tehranifar, A., & Shoor, M. (2013). The use of native turf mixtures to approach sustainable lawn in urban landscapes. Urban Forestry and Urban Greening, 12, 532–536.
Yu, J., Chen, L., Xu, M., et al. (2012). Effects of elevated CO2 on physiological responses of tall fescue to elevated temperature, drought stress, and the combined stresses. Crop Science, 52, 1848–1858. https://doi.org/10.2135/cropsci2012.01.0030.
Silva, F. A. B., Costa, C. M. Q., Moura, R. C., et al. (2010). Study of the dung beetle (Coleoptera: Scarabaeidae) community at two sites: Atlantic forest and clear-cut, Pernambuco, Brazil. Environmental Entomology, 39, 359–367. https://doi.org/10.1603/EN09180.
Barden, S. A. (2011). Red imported fire ant influences on white grub populations and soil foraging characteristics in managed turfgrass. MS thesis, Auburn University, Auburn, Alabama.
Chen, R. Z., Klein, M. G., Sheng, C. F., et al. (2013). Male and female Popillia quadriguttata (Fabricius) and Protaetia brevitarsis (Lewis) (Coleoptera: Scarabaeidae) response to Japanese beetle floral and pheromone lures. Journal of Asia-Pacific Entomology, 16, 479–484. https://doi.org/10.1016/j.aspen.2013.08.001.
Mir Saeedi, H. (2011). Study of some biological traits and seasonal changes of Pentadon idiota Herbest in Shiraz city and the effect of Entonema® and Larvanema® on its larvae in greenhouse conditions. MS thesis, Shiraz University, Shiraz. Iran.
Zhang, W. J. (2018). Global pesticide use: Profile, trend, cost/benefit and more. International Academy of Ecology and Environmental Sciences, 8, 1–27.
Arthurs, S. P., & Bruck, D. J. (2017). Microbial control of nursery ornamental and landscape plant pests. Microbial Control of Insect and Mite Pests, 24, 355–366. https://doi.org/10.1016/B978-0-12-803527-6.00024-X.
Liesch, P. J., & Williamson, R. C. (2010). Evaluation of chemical controls and entomopathogenic nematodes for control of Phyllophaga white grubs in a Fraser fir production field. Journal of Economic Entomology, 103, 1979–1987. https://doi.org/10.1603/EC10176.
Redmond, C. T., & Potter, D. A. (2010). Incidence of turf-damaging white grubs (Coleoptera: Scarabaeidae) and associated pathogens and parasitoids on Kentucky golf courses. Environmental Entomology, 39, 38–47. https://doi.org/10.1603/EN10172.
Liang, L., Tang, S., & Cheke, R. A. (2016). Pure Bt-crop and mixed seed sowing strategies for optimal economic profit in the face of pest resistance to pesticides and Bt-corn. Applied Mathematics and Computation Journal, 283, 6–21. https://doi.org/10.1016/j.amc.2016.02.023.
Sanahuja, G., Banakar, R., Twyman, R. M., et al. (2011). Bacillus thuringiensis: A century of research, development and commercial applications. Plant Biotechnology Journal, 9, 283–300. https://doi.org/10.1111/j.1467-7652.2011.00595.x.
Sampoux, J. P., Baudouin, P., Bayle, B., et al. (2012). Breeding perennial ryegrass (Lolium perenne L.) for turf usage: an assess-ment of genetic improvements in cultivars released in Europe, 1974–2004. Grass and Forage Science, 68, 33–48. https://doi.org/10.1111/j.1365-2494.2012.00896.x.
Humphreys, M., Feuerstein, U., Vandewalle, M., et al. (2010). Ryegrass. In B. Boller, U. K. Posselt, & F. Veronesi (Eds.), Fodder crops and amenity grasses (pp. 211–260). New York: Springer.
Wu, Y. D. (2014). Detection and mechanisms of resistance evolved in insects to cry toxins from Bacillus thuringiensis. Advances in Insect Physiology, 47, 297–342. https://doi.org/10.1016/B978-0-12-800197-4.00006-3.
Yamaguchi, T., Sahara, K., Bando, H., et al. (2008). Discovery of a novel Bacillus thuringiensis cry8D protein and the unique toxicity of the cry8D-class proteins against scarab beetles. Journal of Invertebrate Pathology, 99, 257–262. https://doi.org/10.1016/j.jip.2008.05.009.
Adang, M., Crickmore, N., & Jurat-Fuentes, J. L. (2014). Diversity of Bacillus thuringiensis crystal toxins and mechanism of action. In T. S. Dhadialla & S. Gill (Eds.), Advances in insect physiology (pp. 39–87). Oxford: Academic Press.
Barfoot, P., & Brookes, G. (2014). Key global environmental impacts of genetically modified (GM) crop use 1996–2012. GM Crops and Food, 5, 149–160. https://doi.org/10.4161/gmcr.28449.
Salehi, H., & Kosh-Khui, M. (2005). Effects of genotype and plant growth regulator on callus induction and plant regeneration in four important turfgrass genera: A comparative study. Vitro Cellular and Developmental Biology Plant, 41, 157–161. https://doi.org/10.1079/IVP2004614.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum, 15, 473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x.
Jefferson, R. A. (1987). Assaying chimeric gene in plants: The GUS gene fusion system. Plant Molecular Biology Reporter, 5, 387–405. https://doi.org/10.1007/BF02667740.
Doyle, J. J., & Doyle, J. L. (1990). Isolation of plant DNA from fresh tissue. Focus, 12, 13–15.
Perrot-Rechenmann, C. (2010). Cellular responses to auxin: Division versus expansion. Cold Spring Harbor Perspectives in Biology, 2, 1–15. https://doi.org/10.1101/cshperspect.a001446.
Salehi, H., Salehi, M. R., & Sticklen, M. B. (2008). Tissue culture and genetic transformation of some turfgrass genera. Floriculture, Ornamental and Plant Biotechnology, 2, 25–31.
Krans, J. V., Henning, V. T., & Torres, K. C. (1982). Callus induction, maintenance and plantlet regeneration in creeping bentgrass. Crop Science, 22, 1193–1197. https://doi.org/10.2135/cropsci1982.0011183X002200060025x.
Bai, Y. (2001). Tissue culture and genetic transformation of tall fescue. PhD thesis, North Carolina State University, NC, USA.
Lee, S. H., Lee, D. G., & Lee, B. H. (2004). Effects of medium supplements on seed-derived callus culture and regeneration of orchardgrass. Korean Journal of Crop Science, 49, 232–236. https://doi.org/10.5187/JAST.2004.46.2.243.
Danilova, S. A., & Dolgikh, Y. I. (2005). Optimization of Agrobacterial (Agrobacterium tumefaciens) transformation of maize embryogenic callus. Russian Journal of Plant Physiology, 4, 535–541. https://doi.org/10.1007/s11183-005-0079-5.
Salehi, H., Seddighi, Z., Kravchenko, A. N., et al. (2005). Expression of the CRY1AC in ،Arizona common, common Bermudagrass via Agrobacterium mediated transformation and control of black cutworm. Journal of the American Society for Horticultural Science, 130, 619–623. https://doi.org/10.21273/JASHS.130.4.619.
Ogaki, M., Furuichi, Y., Kuroda, K., et al. (2008). Importance of co-cultivation medium pH for successful Agrobacterium-mediated transformation of Lilium × formolongi. Plant Cell Reports, 27, 699–705. https://doi.org/10.1007/s00299-007-0481-x.
Azadi, P., Chin, D. P., Kuroda, K., et al. (2010). Macro elements in inoculation and co-cultivation medium strongly affect the efficiency of Agrobacterium-mediated transformation in Lilium. Plant Cell Tissue Organ Culture, 101, 201–209. https://doi.org/10.1007/s11240-010-9677-9.
Montoro, P., Teinseree, N., Rattana, W., et al. (2000). Effect of exogenous calcium on Agrobacterium tumefaciens-mediated gene transfer in Hevea brasiliensis (rubber tree) friable calli. Plant Cell Reports, 19, 851–855. https://doi.org/10.1007/s002990000208.
Hoshi, Y., Kondo, M., Mori, S., et al. (2004). Production of transgenic lily plants by Agrobacterium-mediated transformation. Plant Cell Reports, 22, 359–364. https://doi.org/10.1007/s00299-003-0700-z.
Dupre, P., Lacoux, J., Neutelings, G., et al. (2000). Genetic transformation of Ginkgo biloba by Agrobacterium tumefaciens. Physiologia Plantarum, 108, 413–419. https://doi.org/10.1034/j.1399-3054.2000.t01-1-100411.x.
Ankenbauer, R. G., & Nester, E. W. (1990). Sugar-mediated induction of Agrobacterium tumefaciens virulence genes: structural specificity and activities of monosaccharides. Journal of Bacteriology, 172, 6442–6446. https://doi.org/10.1128/jb.172.11.6442-6446.1990.
Shimoda, N., Toyoda-Yamamoto, A., Nagamine, J., et al. (1990). Control of expression of Agrobacterium VIR genes by synergistic actions of phenolic signal molecules and monosaccharides. Proceedings of the National Academy of Sciences USA, 87, 6684–6688. https://doi.org/10.1073/pnas.87.17.6684.
Ahn, J. H., & Lee, J. S. (2003). Sugar acts as a regulatory signal on the wound-inducible expression of SbHRGP3:GUS in transgenic plants. Plant Cell Reports, 22, 286–293. https://doi.org/10.1007/s00299-003-0685-7.
Wise, A. A., Voinov, L., & Binns, A. N. (2005). Intersubunit complementation of sugar signal transduction in VIRA heterodimers and posttranslational regulation of VIRA activity in Agrobacterium tumefaciens. Journal of Bacteriology, 187, 213–223. https://doi.org/10.1128/JB.187.1.213-223.2005.
Ahmad, A., Maqbool, S. B., Riazuddin, S., et al. (2002). Expression of synthetic CRY1AB and CRY1AC genes in Basmati rice (Oryza sativa L.) variety 370 via Agrobacterium-mediated transformation for the control of the european corn borer (Ostrinia nubilalis). Vitro Cellular and Developmental Biology Plant, 38, 213–220. https://doi.org/10.1079/IVPIVP2001277.
Luciani, G., Altpeter, F., Yactayo-Chang, J., et al. (2007). Expression of CRY1FA in Bahiagrass enhances resistance to fall armyworm. Crop Science, 47, 2430–2436. https://doi.org/10.2135/cropsci2007.04.0195.
Shu, C. L., Yan, G., Wang, R. Y., et al. (2009). Characterization of a novel CRY8 gene specific to Melolonthidae pests: Holotrichia oblita and Holotrichia parallela. Applied Microbiology and Biotechnology, 84, 701–707. https://doi.org/10.1007/s00253-009-1971-2.
Shu, C. L., Liu, R. M., Wang, R. Y., et al. (2007). Improving toxicity of Bacillus thuringiensis strain contains the CRY8CA gene specific to metallic green beetle (Anomala corpulenta Motschulsky.) larvae. Current Microbiology, 55, 492–496.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hosseini, H.R., Salehi, H. & Alichi, M. Acquirement of CRY8DB Transgenic Tall Fescue (Festuca arundinacea Schreb.) by Agrobacterium tumefaciens to Develop Resistance Against Pentodon idiota Herbest.. Mol Biotechnol 61, 528–540 (2019). https://doi.org/10.1007/s12033-019-00183-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-019-00183-5