Abstract
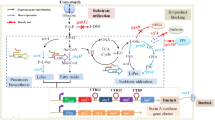
Urea in alcoholic beverage is a precursor of ethyl carbamate (EC), which is carcinogenic. Enzymatic elimination of urea has attracted much research interest. Acid urease with good tolerance toward ethanol and acid is ideal enzyme for such applications. In the present work, the structural genes of urease from Providencia rettgeri JN-B815, ureABC were efficiently expressed in E. coli BL21(DE3) in an active form (apourease) exhibiting both urease and urethanase (hydrolyze EC) activities. The specific activities of the purified apourease were comparatively low, which were 2.1 U/mg for urease and 0.6 U/mg for urethanase, respectively. However, apourease exhibited good resistance toward ethanol and acidic conditions. The relative activities of urease and urethanase remained over 80% in the buffers within pH 4–7. And the recoveries of both urease and urethanase activities were more than 50% in 5–25% ethanol solution. Apourease was utilized to eliminate urea in wine, and the residual urea in model wine was less than 50% after treatment with apourease for 30 h. Then 3D structure of UreC was predicted, and it was docked with urea and EC, respectively. The docking result revealed that three hydrogen bonds were formed between urea and amino acid residues in the active site of urease, whereas only one hydrogen bond can be formed between EC and the active center. Moreover, EC exhibited greater steric hindrance than urea when combined with the active site. Due to the low specific activities of apourease, both structural genes and accessory genes of urease were co-expressed in E. coli BL21(DE3). The holoenzyme was expressed as inclusion body. After renaturation and purification, the specific activities of urease and urethanase reached 10.7 and 3.8 U/mg, which were 5.62-fold and 6.33-fold of those of apourease, respectively. Therefore, accessory subunits of urease play an important role in enhancing urease and urethanase activities.










Similar content being viewed by others
References
Ough, C. S. (1976). Ethyl carbamate in fermented beverages and foods. I. Naturally occurring ethylcarbamate. Journal of Agricultural and Food Chemistry, 24, 323–328.
Witte, C.-P. (2011). Urea metabolism in plants. Plant Science, 180, 431–438.
Mobley, H. L., & Hausinger, R. P. (1989). Microbial ureases: Significance, regulation, and molecular characterization. Microbiological Reviews, 53, 85–108.
Krajewska, B. (2009). Ureases I. Functional, catalytic and kinetic properties: A review. Journal of Molecular Catalysis. B, Enzymatic, 59, 9–21.
Zambelli, B., Musiani, F., Benini, S., & Ciurli, S. (2011). Chemistry of Ni2 + in urease: Sensing, trafficking, and catalysis. Accounts of Chemical Research, 44, 520–530.
Mcmillan, D. J., Mau, M., & Walker, M. J. (1998). Characterisation of the urease gene cluster in Bordetella bronchiseptica. Gene, 208, 243–251.
Yang, Y., Kang, Z., Zhou, J., Chen, J., & Du, G. (2015). High-level expression and characterization of recombinant acid urease for enzymatic degradation of urea in rice wine. Applied Microbiology and Biotechnology, 99, 301–308.
Mulrooney, S. B., & Hausinger, R. P. (1990). Sequence of the Klebsiella aerogenesurease genes and evidence for accessory proteins facilitating nickel incorporation. Journal of Bacteriology, 172, 5837–5843.
Cussac, V., Ferrero, R. L., & Labigne, A. (1992). Expression of Helicobacter pylori urease genes in Escherichia coli grown under nitrogen-limiting conditions. Journal of Bacteriology, 174, 2466–2473.
Lee, M. H., Mulrooney, S. B., Renner, M. J., Markowicz, Y., & Hausinger, R. P. (1992). Klebsiella aerogenes urease gene cluster: Sequence of ureD and demonstration that four accessory genes (ureD, ureE, ureF, and ureG) are involved in nickel metallocenter biosynthesis. Journal of Bacteriology, 174, 4324–4330.
Park, I. S., & Hausinger, R. P. (1993). Site-directed mutagenesis of Klebsiella aerogenes urease: Identification of histidine residues that appear to function in nickel ligation, substrate binding, and catalysis. Protein Science A Publication of the Protein Society, 2, 1034–1041.
Park, I. S., Carr, M. B., & Hausinger, R. P. (1994). In vitro activation of apourease and role of UreD as a chaperone required for nickel metallocenter assembly. Proceedings of the National Academy of Sciences, 91, 3233–3237.
Mobley, H. L. T., & Island, M. D. (1995). Molecular biology of microbial ureases. Microbiological Reviews, 59, 451.
Park, I. S., & Hausinger, R. P. (1995). Evidence for the presence of apourease complexes containing UreD, UreF, and UreG in cells that are competent for in vivo enzyme activation. Journal of Bacteriology, 177, 1947–1951.
Kakimoto, S., Sumino, Y., Kawahara, K., Yamazaki, E., & Nakatsui, I. (1998). Purification and characterization of acid urease from Lactobacillus fermentum. Biophysical Journal, 46, 349–353.
Yamazaki, E., Kurasawa, T., Kakimoto, S., Sumino, Y., & Nakatsui, I. (1990). Characteristics of acid urease from Streptococcus mitior. Agricultural and Biological Chemistry, 54, 2433–2435.
Miyagawa, K., Sumida, M., Nakao, M., Harada, M., Yamamoto, H., Kusumi, T., et al. (1999). Purification, characterization, and application of an acid urease from Arthrobacter mobilis. Royal Society of London Proceedings, 68, 227–236.
Zhang, Q., Zha, X., Zhou, N., & Tian, Y. (2015). Preparation of crosslinked enzyme aggregates (CLEAs) of acid urease with urethanase activity and their application. Journal of Basic Microbiology, 56(4), 422–431.
Kane, J. F., & Hartley, D. L. (1988). Formation of recombinant protein inclusion bodies in Escherichia coli. Trends in Biotechnology, 6, 95–101.
Hu, L. T., & Mobley, H. L. (1993). Expression of catalytically active recombinant Helicobacter pylori urease at wild-type levels in Escherichia coli. Infection and Imumunity, 61, 2563–2569.
Kim, J. K., Mulrooney, S. B., & Hausinger, R. P. (2005). Biosynthesis of active Bacillus subtilis urease in the absence of known urease accessory proteins. Journal of Bacteriology, 187, 7150–7154.
Middelberg, A. P. J. (2002). Preparative protein refolding. Trends in Biotechnology, 20, 437–443.
De Bernardez Clark, E. (1998). Refolding of recombinant proteins. Current Opinion in Biotechnology, 9, 157–163.
Tsumoto, K., Ejima, D., Kumagai, I., & Arakawa, T. (2003). Practical considerations in refolding proteins from inclusion bodies. Protein Expression and Purification, 28, 1–8.
Weatherburn, M. W. (1967). Phenol-hypochlorite reaction for determination of ammonia. Analytical Chemistry, 39(8), 971–974.
Bordoli, L., Kiefer, F., Arnold, K., Benkert, P., Battey, J., & Schwede, T. (2008). Protein structure homology modeling using SWISS-MODEL workspace. Nature Protocols, 4, 1–13.
Luthy, R., Bowie, J. U., & Eisenberg, D. (1992). Assessment of protein models with three-dimensional profiles. Nature, 356, 83–85.
Hui, S. L., & Zhang, Y. (2012). BSP-SLIM: A blind low-resolution ligand-protein docking approach using predicted protein structures. Proceedings of the National Academy of Sciences, 80, 93–110.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Benini, S., Kosikowska, P., Cianci, M., Mazzei, L., Vara, A. G., Berlicki, Ł., et al. (2013). The crystal structure of Sporosarcina pasteurii urease in a complex with citrate provides new hints for inhibitor design. JBIC Journal of Biological Inorganic Chemistry, 18, 391–399.
Ferrer, M., Chernikova, T. N., Yakimov, M. M., Golyshin, P. N., & Timmis, K. N. (2003). Chaperonins govern growth of Escherichia coli at low temperatures. Nature Biotechnology, 21, 1266–1267.
Balagurunathan, B., & Jayaraman, G. (2008). Cellular response to accumulation of recombinant proteins in the E. coli inner membrane: Implications for proteolysis and productivity of the secretory expression system. Biochemical Engineering Journal, 39, 74–83.
William, E. B., Mirjalili, N., Andersen, D. C., Davis, R. H., & Kompala, D. S. (1990). Plasmid-encoded protein: The principal factor in the “metabolic burden” associated with recombinant bacteria. Biotechnology and Bioengineering, 35, 668–681.
Benoit, S. L., & Maier, R. J. (2011). Mua (HP0868) is a nickel-binding protein that modulates urease activity in Helicobacter pylori. Mbio, 2(2), 00039-11.
Mazzei, L., Cianci, M., Benini, S., Bertini, L., Musiani, F., & Ciuli, S. (2015). Kinetic and structural studies reveal a unique binding mode of sulfite to the nickel center in Urease. Journal of Biological Inorganic Chemistry, 154, 42–49.
Benini, S., Kosikowska, P., Cianci, M., Mazzei, L., Vara, A. G., Berlicki, L., et al. (2013). The crystal structure of Sporosarcina Pasteurii urease in a complex with citrate provides new hints for Inhibitor design. Journal of Biological Inorganic Chemistry, 18, 391–399.
Boer, J. L., & Hausinger, R. P. (2012). Klebsiella aerogenes ureF: Identification of the ureG binding site and role in enhancing the fidelity of urease activation. Biochemistry, 51(11), 2298–2308.
Singh, A., Panting, R. J., Varma, A., Saijo, T., Waldron, K. J., Jong, A., et al. (2013). Factors required for activation of urease as a virulence determinant in cryptococcus neoformans. Mbio, 4(3), 00220-13.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31271860), the Joint Innovation Fund of Jiangsu Province (BY2014023-22), the Program for New Century Excellent Talents in University (NCET-12-0878) and the Fundamental Research Funds for the Central Universities (JUSRP51402A). There were no financial/commercial conflicts of interest in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, X., Zhang, Q., Zhou, N. et al. Expression of an Acid Urease with Urethanase Activity in E. coli and Analysis of Urease Gene. Mol Biotechnol 59, 84–97 (2017). https://doi.org/10.1007/s12033-017-9994-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-017-9994-x