Abstract
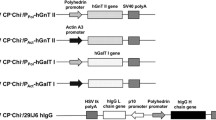
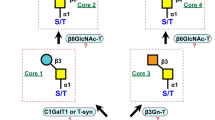
Baculovirus expression vector system (BEVS) is widely known as a mass-production tool to produce functional recombinant glycoproteins except that it may not be always suitable for medical practice due to the differences in the structure of N-linked glycans between insects and mammalian. Currently, various approaches have been reported to alter N-linked glycan structures of glycoproteins derived from insects into terminally sialylated complex-type N-glycans. In the light of those studies, we also proposed in vitro maturation of N-glycan with mass-produced and purified glycosyltransferases by silkworm–BEVS. β-1,4-Galactosyltransferase 1 (β4GalT1) is known as one of type II transmembrane enzymes that transfer galactose in a β-1, 4 linkage to accepter sugars, and a key enzyme for further sialylation of N-glycans. In this study, we developed a large-scale production of recombinant human β4GalT1 (rhβ4GalT1) with N- or C-terminal tags in silkworm–BEVS. We demonstrated that rhβ4GalT1 is N-glycosylated and without mucin-type glycosylation. Interestingly, we found that purified rhβ4GalT1 from silkworm serum presented higher galactosyltransferase activity than that expressed from cultured mammalian cells. We also validated the UDP-galactose transferase activity of produced rhβ4GalT1 proteins by using protein subtracts from silkworm silk gland. Taken together, rhβ4GalT1 from silkworms can become a valuable tool for producing high-quality recombinant glycoproteins with mammalian-like N-glycans.




Similar content being viewed by others
References
Sackstein, R., Merzaban, J. S., Cain, D. W., Dagia, N. M., Spencer, J. A., Lin, C. P., et al. (2008). Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nature Medicine, 14(2), 181–187.
Merzaban, J. S., Imitola, J., Starossom, S. C., Zhu, B., Wang, Y., Lee, J., et al. (2015). Cell surface glycan engineering of neural stem cells augments neurotropism and improves recovery in a murine model of multiple sclerosis. Glycobiology, 25(12), 1392–1409.
Sackstein, R. (2016). Fulfilling Koch’s postulates in glycoscience: HCELL, GPS and translational glycobiology. Glycobiology, 26(6), 560–570.
Hidalgo, A., Weiss, L. A., & Frenette, P. S. (2002). Functional selectin ligands mediating human CD34(+) cell interactions with bone marrow endothelium are enhanced postnatally. The Journal of Clinical Investigation, 110(4), 559–569.
Xia, L., McDaniel, J. M., Yago, T., Doeden, A., & McEver, R. P. (2004). Surface fucosylation of human cord blood cells augments binding to P-selectin and E-selectin and enhances engraftment in bone marrow. Blood, 104(10), 3091–3096.
Robinson, S. N., Simmons, P. J., Thomas, M. W., Brouard, N., Javni, J. A., Trilok, S., et al. (2012). Ex vivo fucosylation improves human cord blood engraftment in NOD-SCID IL-2Rgamma(null) mice. Experimental Hematology, 40(6), 445–456.
Wan, X., Sato, H., Miyaji, H., McDaniel, J. M., Wang, Y., Kaneko, E., et al. (2013). Fucosyltransferase VII improves the function of selectin ligands on cord blood hematopoietic stem cells. Glycobiology, 23(10), 1184–1191.
Masri, K. A., Appert, H. E., & Fukuda, M. N. (1988). Identification of the full-length coding sequence for human galactosyltransferase (β-N-acetylglucosaminide: β1,4-galactosyltransferase). Biochemical and Biophysical Research Communications, 157(2), 657–663.
Aoki, D., Appert, H. E., Johnson, D., Wong, S. S., & Fukuda, M. N. (1990). Analysis of the substrate binding sites of human galactosyltransferase by protein engineering. The EMBO Journal, 9(10), 3171–3178.
Lopez, L. C., Youakim, A., Evans, S. C., & Shur, B. D. (1991). Evidence for a molecular distinction between Golgi and cell surface forms of β1,4-galactosyltransferase. Journal of Biological Chemistry, 266(24), 15984–15991.
Jarvis, D. L. (2003). Developing baculovirus-insect cell expression systems for humanized recombinant glycoprotein production. Virology, 310(1), 1–7.
Harrison, R. L., & Jarvis, D. L. (2006). Protein N-glycosylation in the baculovirus-insect cell expression system and engineering of insect cells to produce “mammalianized” recombinant glycoproteins. Advances in Virus Research, 68, 159–191.
Toth, A. M., Kuo, C. W., Khoo, K. H., & Jarvis, D. L. (2014). A new insect cell glycoengineering approach provides baculovirus-inducible glycogene expression and increases human-type glycosylation efficiency. Journal of Biotechnology, 182–183(1), 19–29.
Geisler, C., Mabashi-Asazuma, H., Kuo, C. W., Khoo, K. H., & Jarvis, D. L. (2015). Engineering β1,4-galactosyltransferase I to reduce secretion and enhance N-glycan elongation in insect cells. Journal of Biotechnology, 193, 52–65.
Seo, N., Hollister, J. R., & Jarvis, D. L. (2001). Mammalian glycosyltransferase expression allows sialoglycoprotein production by baculovirus-infected insect cells. Protein Expression and Purification, 241, 234–241.
Summers, M. D. (2006). Milestones leading to the genetic engineering of baculoviruses as expression vector systems and viral pesticides. Advances in Virus Research, 68(06), 3–73.
Kato, T., Kajikawa, M., Maenaka, K., & Park, E. Y. (2010). Silkworm expression system as a platform technology in life science. Applied Microbiology and Biotechnology, 85(3), 459–470.
Tauber, P., Krammer, F., Palmberger, D., Rendi, D., Wilson, I. B. H., & Grabherr, R. (2011). Insect cells for antibody production: Evaluation of an efficient alternative. Journal of Biotechnology, 153, 160–166.
Aumiller, J. J., Mabashi-asazuma, H., Hillar, A., Shi, X., & Jarvis, D. L. (2012). A new glycoengineered insect cell line with an inducibly mammalianized protein N-glycosylation pathway. Glycobiology, 22(3), 417–428.
Lee, J. M., Kawakami, N., Mon, H., Mitsunobu, H., Iiyama, K., Ninaki, S., et al. (2012). Establishment of a Bombyx mori nucleopolyhedrovirus (BmNPV) hyper-sensitive cell line from the silkworm e21 strain. Biotechnology Letters, 34, 1–7.
Ono, C., Nakatsukasa, T., Nishijima, Y., Asano, S., Sahara, K., & Bando, H. (2007). Construction of the BmNPV T3 bacmid system and its application to the functional analysis of BmNPV he65. Journal of Insect Biotechnology and Sericology, 167, 161–167.
Morokuma, D., Xu, J., Mon, H., Hirata, K., Hino, M., Kuboe, S., et al. (2015). Human alpha 1-acid glycoprotein as a model protein for glycoanalysis in baculovirus expression vector system. Journal of Asia-Pacific Entomology, 18(2), 303–309.
Soejima, Y., Lee, J. M., Nagata, Y., Mon, H., Iiyama, K., Kitano, H., et al. (2013). Comparison of signal peptides for efficient protein secretion in the baculovirus-silkworm system. Central European Journal of Biology, 8(1), 1–7.
Funita-Yamaguchi, Y., & Yoshida, A. (1981). Purification and characterization of human serum galactosyltransferase (lactose synthetase A protein). Journal of Biological Chemistry, 256(6), 2701–2706.
Kim, Y. K., Kim, K. R., Kang, D. G., Jang, S. Y., Kim, Y. H., & Cha, H. J. (2009). Suppression of β-N-acetylglucosaminidase in the N-glycosylation pathway for complex glycoprotein formation in Drosophila S2 cells. Glycobiology, 19(3), 301–308.
Iizuka, M., Ogawa, S., Takeuchi, A., Nakakita, S., Kubo, Y., Miyawaki, Y., et al. (2009). Production of a recombinant mouse monoclonal antibody in transgenic silkworm cocoons. FEBS Journal, 276(20), 5806–5820.
Marchal, I., Jarvis, D. L., Cacan, R., & Verbert, A. (2001). Glycoproteins from insect cells: Sialylated or not? Biological Chemistry, 382(2), 151–159.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morokuma, D., Xu, J., Hino, M. et al. Expression and Characterization of Human β-1, 4-Galactosyltransferase 1 (β4GalT1) Using Silkworm–Baculovirus Expression System. Mol Biotechnol 59, 151–158 (2017). https://doi.org/10.1007/s12033-017-0003-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-017-0003-1