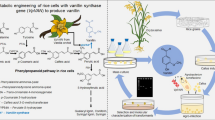
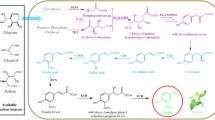
Abstract
Production of vanillin by bioengineering has gained popularity due to consumer demand toward vanillin produced by biological systems. Natural vanillin from vanilla beans is very expensive to produce compared to its synthetic counterpart. Current bioengineering works mainly involve microbial biotechnology. Therefore, alternative means to the current approaches are constantly being explored. This work describes the use of vanillin synthase (VpVAN), to bioconvert ferulic acid to vanillin in a plant system. The VpVAN enzyme had been shown to directly convert ferulic acid and its glucoside into vanillin and its glucoside, respectively. As the ferulic acid precursor and vanillin were found to be the intermediates in the phenylpropanoid biosynthetic pathway of Capsicum species, this work serves as a proof-of-concept for vanillin production using Capsicum frutescens (C. frutescens or hot chili pepper). The cells of C. frutescens were genetically transformed with a codon optimized VpVAN gene via biolistics. Transformed explants were selected and regenerated into callus. Successful integration of the gene cassette into the plant genome was confirmed by polymerase chain reaction. High-performance liquid chromatography was used to quantify the phenolic compounds detected in the callus tissues. The vanillin content of transformed calli was 0.057% compared to 0.0003% in untransformed calli.







Similar content being viewed by others
References
Rao, S. R., & Ravishankar, G. A. (2000). Vanilla flavor: Production by conventional and biotechnological routes. Journal of the Science of Food and Agriculture, 80, 289–304.
Cheng, W.-Y., Hsiang, C.-Y., Bau, D.-T., Chen, J.-C., Shen, W.-S., Li, C.-C., et al. (2007). Microarray analysis of vanillin-regulated gene expression profile in human hepatocarcinoma cells. Pharmacological Research, 56, 474–482.
Cerrutti, P., Alzamora, S. M., & Vidales, S. L. (1997). Vanillin as an antimicrobial for producing shelf-stable strawberry puree. Journal of Food Science, 62, 608–610.
Market Insider, I. T. C. (2016). Frightening ride forecast for vanilla. International Trade Centre. http://www.intracen.org/itc/blogs/market-insider/Frightening-ride-forecast-for-vanilla/. Retrieved Sept 19, 2016.
Rana, R., Mathur, A., Jain, C. K., Sharma, S. K., & Mathur, G. (2013). Microbial production of vanillin. International Journal of Biotechnology Bioengineering Research, 4, 227–234.
de Melo, J. (2015). Developing countries in the world economy. Singapore: World Scientific.
Sabisch, M. & Smith, D. (2014). The complex regulatory landscape for natural flavor ingredients. Sigma-Aldrich. http://www.sigmaaldrich.com/technical-documents/articles/white-papers/flavors-and-fragrances/natural-flavor-ingredients-regulations.html#definitions. Retrieved Dec 1, 2015.
Converti, A., Aliakbarian, B., Domínguez, J. M., Vazquez, G. B., & Perego, P. (2010). Microbial production of biovanillin. Brazilian Journal of Microbiology, 41, 519–530.
Longo, M. A., & Sanromán, M. A. (2006). Production of food aroma compounds: Microbial and enzymatic methodologies. Food Technology and Biotechnology, 44, 335–353.
Gallage, N. J., Hansen, E. H., Kannangara, R., Olsen, C. E., Motawia, M. S., Jørgensen, K., et al. (2014). Vanillin formation from ferulic acid in Vanilla planifolia is catalysed by a single enzyme. Nature Communications, 5, 4037. doi:10.1038/ncomms5037.
Pometto, A. L., III, & Crawford, D. L. (1983). Whole-cell bioconversion of vanillin to vanillic acid by Streptomyces viridosporus. Applied and Environment Microbiology, 45, 1582–1585.
Narbad, A., & Gasson, M. J. (1998). Metabolism of ferulic acid via vanillin using a novel CoA-dependent pathway in a newly-isolated strain of Pseudomonas fluorescens. Microbiology, 144, 1397–1405.
Hansen, E. H., Møller, B. L., Kock, G. R., Bünner, C. M., Kristensen, C., Jensen, O. R., et al. (2009). De novo biosynthesis of vanillin in fission yeast (Schizosaccharomyces pombe) and baker’s yeast (Saccharomyces cerevisiae). Applied and Environment Microbiology, 75, 2765–2774.
Sukrasno, N., & Yeoman, M. M. (1993). Phenylpropanoid metabolism during growth and development of Capsicum frutescens fruits. Phytochemistry, 32, 839.
Chiu, W. L., Niwa, Y., Zeng, W., Hirano, T., Kobayashi, H., & Sheen, J. (1996). Engineered GFP as a vital reporter in plants. Current Biology, 6, 325–330.
Gallage, N. J., & Møller, B. L. (2015). Vanillin-bioconversion and bioengineering of the most popular plant flavor and its de novo biosynthesis in the vanilla orchid. Molecular Plant, 8, 40–57.
Matthew, S., & Abraham, T. E. (2004). Ferulic acid: An antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Critical Reviews in Biotechnology, 24, 59–83.
Dobberstein, D., & Bunzel, M. (2010). Separation and detection of cell wall-bound ferulic acid dehydrodimers and dehydrotrimers in cereals and other plant materials by reversed phase high-performance liquid chromatography with ultraviolet detection. Journal of Agriculture and Food Chemistry, 58, 8927–8935.
Yahiaoui, N., Marque, C., Myton, K. E., Negrel, J., & Boudet, A. M. (1998). Impact of different levels of cinnamyl alcohol dehydrogenase down-regulation on lignins of transgenic tobacco plants. Planta, 204, 8–15.
Gururaj, H. B., Padma, M. N., Giridhar, P., & Ravishankar, G. A. (2012). Functional validation of Capsicum frutescens aminotransferase gene involved in vanillylamine biosynthesis using Agrobacterium mediated genetic transformation studies in Nicotiana tabacum and Capsicum frutescens calli cultures. Plant Science, 195, 96–105.
Rodas-Junco, B. A., Cab-Guillén, Y., Muñoz-Sánchez, J. A., Vázquez-Flota, F., Monforte-González, M., & Hernández-Sotomayor, S. M. T. (2013). Salicylic acid induces vanillin synthesis through the phospholipid signaling pathway in Capsicum chinense cell cultures. Plant Signaling & Behavior, 8, e26752.
Zhang, X., & Liu, C.-J. (2015). Multifaceted regulations of gateway enzyme phenylalanine ammonia-lyase in the biosynthesis of phenylpropanoids. Molecular Plant, 8, 17–27.
Pilate, G., Dejardin, A., & Leple, J.-C. (2010). Field trials with lignin-modified transgenic trees. In L. Jouanin & C. Lapierre (Eds.), Lignins: Biosynthesis, Biodegradation and Bioengineering. Cambridge: Academic Press.
Harris, P. J., & Trethewey, J. A. K. (2010). The distribution of ester-linked ferulic acid in the cell walls of angiosperms. Phytochemistry Reviews, 9, 19–33.
Acknowledgements
This work was funded by the Malaysian Ministry of Science, Technology and Innovation through the eScience Fund (02-02-12-SF0130), and by the Malaysian Ministry of Education through the MyBrain15 PhD scholarship programme.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
12033_2016_9986_MOESM1_ESM.tif
Fig. S1 Representation of colony PCR of VpVAN gene from thirteen E. coli colonies transformed with pcDNA6.2::(35Sp-VpVAN-V5)ΔccdB expression vector. M—1 kb DNA ladder; (+)—PCR positive control using synthesized holding vector, pIDT-AMP:35Sp-VpVAN-V5, as the template; (−)—no template PCR control. (TIFF 1049 kb)
12033_2016_9986_MOESM2_ESM.tif
Fig. S2 (i) PCR of VpVAN gene from pcDNA6.2::(35Sp-VpVAN-V5)ΔccdB expression vector extracted from E. coli, and (ii) DNA profile of the extracted expression vector after double restriction digest with PstI and BamHI restriction endonucleases (REs). M—1 kb DNA ladder; (+)—PCR positive control using synthesized holding vector, pIDT-AMP:35Sp-VpVAN-V5, as the template; (−)—no template PCR control. (TIFF 1303 kb)
Rights and permissions
About this article
Cite this article
Chee, M.J.Y., Lycett, G.W., Khoo, TJ. et al. Bioengineering of the Plant Culture of Capsicum frutescens with Vanillin Synthase Gene for the Production of Vanillin. Mol Biotechnol 59, 1–8 (2017). https://doi.org/10.1007/s12033-016-9986-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-016-9986-2