Abstract
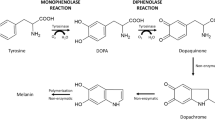
Human tyrosinase is the first enzyme of the multistep process of melanogenesis. It catalyzes the hydroxylation of l-tyrosine to l-dihydroxyphenylalanine and the following oxidation of o-diphenol to the corresponding quinone, l-dopaquinone. In spite of its biomedical relevance, its reactivity is far from being fully understood, mostly because of the lack of a suitable expression system. Indeed, until now, studies on substrates and inhibitors of tyrosinases have been performed in vitro almost exclusively using mushroom or bacterial enzymes. We report on the production of a recombinant human tyrosinase in insect cells (Sf9 line). Engineering the protein, improving cell culture conditions, and setting a suitable purification protocol optimized product yield. The obtained active enzyme was truthfully characterized with a number of substrate and inhibitor molecules. These results were compared to those gained from a parallel analysis of the bacterial (Streptomyces antibioticus) enzyme and those acquired from the literature for mushroom tyrosinase, showing that the reactivity of the human enzyme appears unique and pointing out the great bias introduced when using non-human tyrosinases to measure the inhibitory efficacy of new molecules. The described enzyme is therefore an indispensable paradigm in testing pharmaceutical or cosmetic agents addressing tyrosinase activity.






Similar content being viewed by others
References
Solano, F., Briganti, S., Picardo, M., & Ghanem, G. (2006). Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Research, 19, 550–571.
Gillbro, J., & Olsson, M. (2011). The melanogenesis and mechanisms of skin-lightening agents–existing and new approaches. International Journal of Cosmetic Science, 33, 210–221.
Matoba, Y., Kumagai, T., Yamamoto, A., Yoshitsu, H., & Sugiyama, M. (2006). Crystallographic evidence that the dinuclear copper center of tyrosinase is flexible during catalysis. Journal of Biological Chemistry, 281, 8981–8990.
Sendovski, M., Kanteev, M., Shuster Ben-Yosef, V., Adir, N., & Fishman, A. (2010). Crystallization and preliminary X-ray crystallographic analysis of a bacterial tyrosinase from Bacillus megaterium. Acta Crystallographica, Section F: Structural Biology and Crystallization Communications, 66, 1101–1103.
Ismaya, W. T., Rozeboom, H. J., Weijn, A., Mes, J. J., Fusetti, F., Wichers, H. J., et al. (2011). Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry, 50, 5477–5486.
Fujieda, N., Yabuta, S., Ikeda, T., Oyama, T., Muraki, N., Kurisu, G., et al. (2013). Crystal structures of copper-depleted and copper-bound fungal pro-tyrosinase: insights into endogenous cysteine-dependent copper incorporation. Journal of Biological Chemistry, 288, 22128–22140.
Takahashi, H., & Parsons, P. G. (1992). Rapid and reversible inhibition of tyrosinase activity by glucosidase inhibitors in human melanoma cells. Journal of Investigative Dermatology, 98, 481–487.
Kong, K.H., (2004). Method for mass-producing of a recombinant human tyrosinase in E. coli. Patent WO 2004048560 A1.
Chen, G., Chen, W., Huang, Y., & Jiang, S. (2012). Expression of recombinant mature human tyrosinase from Escherichia coli and exhibition of its activity without phosphorylation or glycosylation. Journal of Agriculture and Food Chemistry, 60, 2838–2843.
Chen, G., Wang, H., & Li, Z., (2005). Human tyrosinase expression carrier and its use. Patent CN 1603417 A.
Dolinska, M. B., Kovaleva, E., Backlund, P., Wingfield, P. T., Brooks, B. P., & Sergeev, Y. V. (2014). Albinism-causing mutations in recombinant human tyrosinase alter intrinsic enzymatic activity. PLoS One, 9, e84494.
Branza-Nichita, N., Negroiu, G., Petrescu, A. J., Garman, E. F., Platt, F. M., Wormald, M. R., et al. (2000). Mutations at critical N-glycosylation sites reduce tyrosinase activity by altering folding and quality control. Journal of Biological Chemistry, 275, 8169–8175.
Wang, N., & Hebert, D. N. (2006). Tyrosinase maturation through the mammalian secretory pathway: Bringing color to life. Pigment Cell Research, 19, 3–18.
Popescu, C. I., Mares, A., Zdrentu, L., Zitzmann, N., Dwek, R. A., & Petrescu, S. M. (2006). Productive folding of tyrosinase ectodomain is controlled by the transmembrane anchor. Journal of Biological Chemistry, 281, 21682–21689.
Ando, H., Kondoh, H., Ichihashi, M., & Hearing, V. J. (2007). Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. Journal of Investigative Dermatology, 127, 751–761.
Cioaca, D., Ghenea, S., Spiridon, L. N., Marin, M., Petrescu, A., & Petrescu, S. M. (2011). C-terminus glycans with critical functional role in the maturation of secretory glycoproteins. PLoS One, 6, e19979.
Bubacco, L., Vijgenboom, E., Gobin, C., Tepper, A. W., Salgado, J., & Canters, G. W. (2000). Kinetic and paramagnetic NMR investigations of the inhibition of Streptomyces antibioticus tyrosinase. Journal of Molecular Catalysis B: Enzymatic, 8, 27–35.
Greggio, E., Bergantino, E., Carter, D., Ahmad, R., Costin, G., Hearing, V. J., et al. (2005). Tyrosinase exacerbates dopamine toxicity but is not genetically associated with Parkinson’s disease. Journal of Neurochemistry, 93, 246–256.
Espín, J. C., Morales, M., García-Ruiz, P. A., Tudela, J., & García-Cánovas, F. (1997). Improvement of a continuous spectrophotometric method for determining the monophenolase and diphenolase activities of mushroom polyphenol oxidase. Journal of Agriculture and Food Chemistry, 45, 1084–1090.
Espín, J. C., Varón, R., Fenoll, L. G., Gilabert, M., García-Ruíz, P. A., Tudela, J., et al. (2000). Kinetic characterization of the substrate specificity and mechanism of mushroom tyrosinase. European Journal of Biochemistry, 267, 1270–1279.
Willers, J., Lucchese, A., Mittelman, A., Dummer, R., & Kanduc, D. (2005). Definition of anti-tyrosinase MAb T311 linear determinant by proteome-based similarity analysis. Experimental Dermatology, 14, 543–550.
Virador, V., Matsunaga, N., Matsunaga, J., Valencia, J., Oldham, R. J., Kameyama, K., et al. (2001). Production of melanocyte-specific antibodies to human melanosomal proteins: Expression patterns in normal human skin and in cutaneous pigmented lesions. Pigment Cell Research, 14, 289–297.
Tsukamoto, K., Jackson, I. J., Urabe, K., Montague, P. M., & Hearing, V. J. (1992). A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO Journal, 11, 519–526.
Bubacco, L., Salgado, J., Tepper, A. W., Vijgenboom, E., & Canters, G. W. (1999). 1H NMR spectroscopy of the binuclear Cu (II) active site of Streptomyces antibioticus tyrosinase. FEBS Letters, 442, 215–220.
Vijayan, E., Husain, I., Ramaiah, A., & Madan, N. C. (1982). Purification of human skin tyrosinase and its protein inhibitor: properties of the enzyme and the mechanism of inhibition by protein. Archives of Biochemistry and Biophysics, 217, 738–747.
Hearing, V. J, Jr. (1987). Mammalian monophenol monooxygenase (tyrosinase): Purification, properties, and reactions catalyzed. Methods in Enzymology, 142, 154–165.
Wittbjer, A., Dahlback, B., Odh, G., Rosengren, A. M., Rosengren, E., & Rorsman, H. (1989). Isolation of human tyrosinase from cultured melanoma cells. Acta Dermato Venereologica, 69, 125–131.
Yurkow, E. J., & Laskin, J. D. (1989). Purification of tyrosinase to homogeneity based on its resistance to sodium dodecyl sulfate-proteinase K digestion. Archives of Biochemistry and Biophysics, 275, 122–129.
Pomerantz, S. H. (1966). The tyrosine hydroxylase activity of mammalian tyrosinase. Journal of Biological Chemistry, 241, 161–168.
Olivares, C., García-Borrón, J. C., & Solano, F. (2002). Identification of active site residues involved in metal cofactor binding and stereospecific substrate recognition in mammalian tyrosinase. Implications to the catalytic cycle. Biochemistry, 41, 679–686.
Jergil, B., Lindbladh, C., Rorsman, H., & Rosengren, E. (1983). Dopa oxidation and tyrosine oxygenation by human melanoma tyrosinase. Acta Dermato Venereologica, 63, 468–475.
Husain, I., Vijayan, E., Ramaiah, A., Pasricha, J., & Madan, N. (1982). Demonstration of tyrosinase in the vitiligo skin of human beings by a sensitive fluorometric method as well as by 14C (U)-l-tyrosine incorporation into melanin. Journal of Investigative Dermatology, 78, 243–252.
Winder, A. J., & Harris, H. (1991). New assays for the tyrosine hydroxylase and dopa oxidase activities of tyrosinase. European Journal of Biochemistry, 198, 317–326.
Eleftherianos, I., & Revenis, C. (2011). Role and importance of phenoloxidase in insect hemostasis. Journal of Innate Immunity, 3, 28–33.
González-Santoyo, I., & Córdoba-Aguilar, A. (2012). Phenoloxidase: A key component of the insect immune system. Entomologia Experimentalis et Applicata, 142, 1–16.
Simmen, T., Schmidt, A., Hunziker, W., & Beermann, F. (1999). The tyrosinase tail mediates sorting to the lysosomal compartment in MDCK cells via a di-leucine and a tyrosine-based signal. Journal of Cell Science, 112(Pt 1), 45–53.
Calvo, P. A., Frank, D. W., Bieler, B. M., Berson, J. F., & Marks, M. S. (1999). A cytoplasmic sequence in human tyrosinase defines a second class of di-leucine-based sorting signals for late endosomal and lysosomal delivery. Journal of Biological Chemistry, 274, 12780–12789.
Bubacco, L., Spinazze, R., Longa, S. D., & Benfatto, M. (2007). X-ray absorption analysis of the active site of Streptomyces antibioticus tyrosinase upon binding of transition state analogue inhibitors. Archives of Biochemistry and Biophysics, 465, 320–327.
Bochot, C., Gouron, A., Bubacco, L., Milet, A., Philouze, C., Réglier, M., et al. (2014). Probing kojic acid binding to tyrosinase enzyme: Insights from a model complex and QM/MM calculations. Chemical Communications, 50, 308–310.
Olivares, C., & Solano, F. (2009). New insights into the active site structure and catalytic mechanism of tyrosinase and its related proteins. Pigment cell & melanoma research, 22, 750–760.
Masamoto, Y., Murata, Y., Baba, K., Shimoishi, Y., Tada, M., & Takahata, K. (2004). Inhibitory effects of esculetin on melanin biosynthesis. Biological and Pharmaceutical Bulletin, 27, 422–425.
Ryazanova, A. D., Alekseev, A. A., & Slepneva, I. A. (2012). The phenylthiourea is a competitive inhibitor of the enzymatic oxidation of DOPA by phenoloxidase. Journal of Enzyme Inhibition and Medicinal Chemistry, 27, 78–83.
Goliĉnik, M., & Stojan, J. (2004). Slow-binding inhibition: A theoretical and practical course for students. Biochemistry and Molecular Biology Education, 32, 228–235.
Shi, Y., Chen, Q., Wang, Q., Song, K., & Qiu, L. (2005). Inhibitory effects of cinnamic acid and its derivatives on the diphenolase activity of mushroom (Agaricus bisporus) tyrosinase. Food Chemistry, 92, 707–712.
Nesterov, A., Zhao, J., Minter, D., Hertel, C., Ma, W., Abeysinghe, P., et al. (2008). 1-(2, 4-Dihydroxyphenyl)-3-(2, 4-dimethoxy-3-methylphenyl) propane, a novel tyrosinase inhibitor with strong depigmenting effects. Chemical & Pharmaceutical Bulletin, 56, 1292–1296.
Gasparetti, C., Nordlund, E., Jänis, J., Buchert, J., & Kruus, K. (2012). Extracellular tyrosinase from the fungus Trichoderma reeseis hows product inhibition and different inhibition mechanism from the intracellular tyrosinase from Agaricus bisporus. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 1824, 598–607.
Gouzi, H., Coradin, T., Delicado, E., Ünal, M., & Benmansour, A. (2010). Inhibition kinetics of Agaricus bisporus (JE Lange) Imbach polyphenol oxidase. Open Enzyme Inhibition Journal, 3, 1–7.
Acknowledgments
We thank Dr. E. Greggio (Department of Biology, University of Padova, Italy) for providing a sample of PMEL (Pigmented Melanoma) cells. This work has been financed by the Assegno di Ricerca (CPDR095797/09) and by the Progetto di Ricerca di Ateneo (CPDA110789/11) from the University of Padova to E.B.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Stefano Fogal and Marcello Carotti have contributed equally to the work.
Rights and permissions
About this article
Cite this article
Fogal, S., Carotti, M., Giaretta, L. et al. Human Tyrosinase Produced in Insect Cells: A Landmark for the Screening of New Drugs Addressing its Activity. Mol Biotechnol 57, 45–57 (2015). https://doi.org/10.1007/s12033-014-9800-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-014-9800-y