Abstract
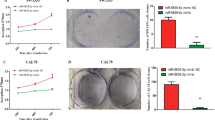
Recent study showed that inflammation was related to lung cancer. However, the exact cause of lung inflammation leading to carcinogenesis is unknown. MicroRNAs (miRNAs) are a group of endogenous non-coding small RNAs that regulate the activity of targeted mRNAs by inflammatory response in many diseases. MiR-451 was reported to relate to the development of lung cancer and metastasis of glioma. But the effect of miR-451 on cell proliferation, migration, and invasion of lung cancer is not really clear. In order to explore the molecular mechanism of the occurrence and development of lung cancer, we investigated the effect of human miR-451 on the proliferation, invasion, and metastasis in lung cancer cell line A549. The miR-451 expression construct was generated into pGenesil-1.1 and transfected into A549 cells. Results showed that the recombinant vectors were verified by sequencing. And miR-451 was over-expressed in A549 by real-time RT PCR. Furthermore, the proliferation, invasion, and metastasis of the cells in miR-451 group were inhibited significantly compared with those in control and A549 groups by MTT assay, Transwell invasion assay, and wound-healing assay. And the lung cancer metastasis factors (MMP-2, MMP-9, VEGF, and CXCR4) were decreased in miR-451 group by Western blot. Moreover, it was proved that inflammation-related gene-PSMB8 was a target for miR-451 by bioinformatics analysis and dual-luciferase reporter assay. And the protein expressions of PSMB8 and NOS2 were decreased in miR-451 group compared with those in control and A549 groups. Therefore, our findings indicated that miR-451 related to PSMB8/NOS2 inflammatory factors may suppress the development and migration of lung cancer, providing evidence for the role of miR-451 in lung cancer.





Similar content being viewed by others
References
Öberg, M., Jaakkola, M. S., Woodward, A., Peruga, A., & Prüss-Ustün, A. (2011). Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. The Lancet, 377(9760), 139–146.
Thill, P. G., Goswami, P., Berchem, G., & Domon, B. (2011). Lung cancer statistics in Luxembourg from 1981 to 2008. Bulletin de la Societe des Sciences Medicales du Grand-Duche de Luxembourg, 2, 43–55.
Sterlacci, W., Fiegl, M., & Tzankov, A. (2012). Prognostic and predictive value of cell cycle deregulation in non-small-cell lung cancer. Pathobiology, 79(4), 175–194.
Hussain, S. P., & Harris, C. C. (2007). Inflammation and cancer: an ancient link with novel potentials. International Journal of Cancer, 121(11), 2373–2380.
Hattar, K., Savai, R., Subtil, F. S., Wilhelm, J., Schmall, A., Lang, D. S., et al. (2013). Endotoxin induces proliferation of NSCLC in vitro and in vivo: role of COX-2 and EGFR activation. Cancer Immunology, Immunotherapy, 62(2), 309–320.
Cho, W. C., Kwan, C. K., Yau, S., So, P. P., Poon, P. C., & Au, J. S. (2011). The role of inflammation in the pathogenesis of lung cancer. Expert opinion on therapeutic targets, 15(9), 1127–1137.
Houghton, A. M., Mouded, M., & Shapiro, S. D. (2008). Common origins of lung cancer and COPD. Nature Medicine, 14(10), 1023–1024.
Lewis, B. P., Burge, C. B., & Bartel, D. P. (2005). Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell, 120(1), 15–20.
Lu, J., Getz, G., Miska, E. A., Alvarez-Saavedra, E., Lamb, J., Peck, D., et al. (2005). MicroRNA expression profiles classify human cancers. Nature, 435(7043), 834–838.
Contreras, J., & Rao, D. (2011). MicroRNAs in inflammation and immune responses. Leukemia, 26(3), 404–413.
Garzon, R., Calin, G. A., & Croce, C. M. (2009). MicroRNAs in cancer. Annual Review of Medicine, 60, 167–179.
Bergamaschi, A., & Katzenellenbogen, B. S. (2012). Tamoxifen downregulation of miR-451 increases 14-3-3ζ and promotes breast cancer cell survival and endocrine resistance. Oncogene, 31(1), 39–47.
Bandres, E., Bitarte, N., Arias, F., Agorreta, J., Fortes, P., Agirre, X., et al. (2009). microRNA-451 regulates macrophage migration inhibitory factor production and proliferation of gastrointestinal cancer cells. Clinical Cancer Research, 15(7), 2281–2290.
Lv G., Hu Z., Tie Y., Du J., Fu H., Gao X., et al. (2014). MicroRNA-451 regulates activating transcription factor 2 expression and inhibits liver cancer cell migration. Oncology Report. doi:10.3892/or.2014.3296.
Zhang, Z., Luo, X., Ding, S., Chen, J., Chen, T., Chen, X., et al. (2012). MicroRNA-451 regulates p38 MAPK signaling by targeting of Ywhaz and suppresses the mesangial hypertrophy in early diabetic nephropathy. FEBS Letters, 586(1), 20–26.
Wang, R., Wang, Z., Yang, J., Pan, X., De, W., & Chen, L. (2011). MicroRNA-451 functions as a tumor suppressor in human non-small cell lung cancer by targeting ras-related protein 14 (RAB14). Oncogene, 30(23), 2644–2658.
Solomides, C. C., Evans, B. J., Navenot, J. M., Vadigepalli, R., Peiper, S. C., & Wang, Z. X. (2012). MicroRNA profiling in lung cancer reveals new molecular markers for diagnosis. Acta Cytologica, 56(6), 645–654.
Markou, A., Sourvinou, I., Vorkas, P., Yousef, G., & Lianidou, E. (2013). Clinical evaluation of microRNA expression profiling in non small cell lung cancer. Lung Cancer, 81(3), 388–396.
Bian, H. B., Pan, X., Yang, J. S., Wang, Z. X., & De, W. (2011). Upregulation of microRNA-451 increases cisplatin sensitivity of non-small cell lung cancer cell line (A549). Journal of Experimental & Clinical Cancer Research, 30(1), 20–31.
Megraw, M., Sethupathy, P., Corda, B., & Hatzigeorgiou, A. G. (2007). miRGen: a database for the study of animal microRNA genomic organization and function. Nucleic Acids Research, 35(suppl 1), D149–D155.
Doench, J. G., Petersen, C. P., & Sharp, P. A. (2003). siRNAs can function as miRNAs. Genes & Development, 17(4), 438–442.
Hua, Z., Lv, Q., Ye, W., Wong, C. K. A., Cai, G., Gu, D., et al. (2006). MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS ONE, 1(1), e116.
Livark, K., & Schmittgen, T. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods, 25(4), 402–408.
Elgamal, O. A., Park, J. K., Gusev, Y., Azevedo-Pouly, A. C. P., Jiang, J., Roopra, A., et al. (2013). Tumor suppressive function of mir-205 in breast cancer is linked to HMGB3 regulation. PLoS ONE, 8(10), e76402.
Humplikova, L., Kollinerova, S., Papajik, T., Pikalova, Z., Holzerova, M., Prochazka, V., et al. (2013). Expression of miR-15a and miR-16-1 in patients with chronic lymphocytic leukemia. Biomedical Papers, 157(4), 284–293.
Peng, W., Chen, Z., Wang, L., Wang, Z., & Li, J. (2013). MicroRNA-199a-3p is downregulated in gastric carcinomas and modulates cell proliferation. Genetics and molecular research: GMR, 12(3), 3038.
Godlewski, J., Nowicki, M. O., Bronisz, A., Nuovo, G., Palatini, J., De Lay, M., et al. (2010). MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Molecular Cell, 37(5), 620–632.
Caudill, C. M., Jayarapu, K., Elenich, L., Monaco, J. J., Colbert, R. A., & Griffin, T. A. (2006). T cells lacking immunoproteasome subunits MECL-1 and LMP7 hyperproliferate in response to polyclonal mitogens. The Journal of Immunology, 176(7), 4075–4082.
Moebius, J., van den Broek, M., Groettrup, M., & Basler, M. (2010). Immunoproteasomes are essential for survival and expansion of T cells in virus-infected mice. European Journal of Immunology, 40(12), 3439–3449.
Singh, A. V., Bandi, M., Aujay, M. A., Kirk, C. J., Hark, D. E., Raje, N., et al. (2011). PR-924, a selective inhibitor of the immunoproteasome subunit LMP-7, blocks multiple myeloma cell growth both in vitro and in vivo. British Journal of Haematology, 152(2), 155–163.
Song, L., Ma, N., Han, L., Yan, H., Yan, B., Yuan, Z., et al. (2013). Association between LMP2/LMP7 genetic variability and the metastasis risk of ovarian cancer in Chinese women in Beijing. Human Immunology, 75(3), 239–244.
Fellerhoff, B., Gu, S., Laumbacher, B., Nerlich, A. G., Weiss, E. H., Glas, J., et al. (2011). The LMP7-K allele of the immunoproteasome exhibits reduced transcript stability and predicts high risk of colon cancer. Cancer Research, 71(23), 7145–7154.
Zheng, F., Hasim, A., Anwer, J., Niyaz, M., & Sheyhidin, I. (2013). LMP gene promoter hypermethylation is a mechanism for its down regulation in Kazak’s esophageal squamous cell carcinomas. Molecular Biology Reports, 40(3), 2069–2075.
Shen, Y. Q., Zhang, J. Q., Xia, M., Miao, F. Q., Shan, X. N., & Xie, W. (2007). Low-molecular-weight protein (LMP) 2/LMP7 abnormality underlies the downregulation of human leukocyte antigen class I antigen in a hepatocellular carcinoma cell line. Journal of Gastroenterology and Hepatology, 22(7), 1155–1161.
Visekruna, A., Joeris, T., Schmidt, N., Lawrenz, M., Ritz, J. P., Buhr, H. J., et al. (2009). Comparative expression analysis and characterization of 20S proteasomes in human intestinal tissues: the proteasome pattern as diagnostic tool for IBD patients. Inflammatory Bowel Diseases, 15(4), 526–533.
Visekruna, A., Joeris, T., Seidel, D., Kroesen, A., Loddenkemper, C., Zeitz, M., et al. (2006). Proteasome-mediated degradation of IkappaBalpha and processing of p105 in Crohn disease and ulcerative colitis. The Journal of Clinical Investigation, 116(12), 3195–3203.
Schmidt, N., Gonzalez, E., Visekruna, A. A., Loddenkemper, C., Mollenkopf, H., Kaufmann, S. H., et al. (2010). Targeting the proteasome: partial inhibition of the proteasome by bortezomib or deletion of the immunosubunit LMP7 attenuates experimental colitis. Gut, 59(7), 896–906.
Muchamuel, T., Basler, M., Aujay, M. A., Suzuki, E., Kalim, K. W., Lauer, C., et al. (2009). A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nature Medicine, 15(7), 781–787.
Kotamraju, S., Matalon, S., Matsunaga, T., Shang, T., Hickman-Davis, J. M., & Kalyanaraman, B. (2006). Upregulation of immunoproteasomes by nitric oxide: potential antioxidative mechanism in endothelial cells. Free Radical Biology and Medicine, 40(6), 1034–1044.
Qureshi, A. A., Tan, X., Reis, J. C., Badr, M. Z., Papasian, C. J., Morrison, D. C., et al. (2011). Suppression of nitric oxide induction and pro-inflammatory cytokines by novel proteasome inhibitors in various experimental models. Lipids in health and disease, 10(1), 177.
Lee, S. H., Jaganath, I. B., Manikam, R., & Sekaran, S. D. (2013). Inhibition of Raf-MEK-ERK and Hypoxia pathways by Phyllanthus prevents metastasis in human lung (A549) cancer cell line. BMC complementary and alternative medicine, 13(1), 271.
Marrogi, A. J., Travis, W. D., Welsh, J. A., Khan, M. A., Rahim, H., Tazelaar, H., et al. (2000). Nitric oxide synthase, cyclooxygenase 2, and vascular endothelial growth factor in the angiogenesis of non-small cell lung carcinoma. Clinical Cancer Research, 6(12), 4739–4744.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81270912) and the project of Science and Technology Commission Foundation of Chongqing Province (cstc2013jcyjA10044).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Pin Yin and Rui Peng are co-first authors.
Rights and permissions
About this article
Cite this article
Yin, P., Peng, R., Peng, H. et al. MiR-451 Suppresses Cell Proliferation and Metastasis in A549 Lung Cancer Cells. Mol Biotechnol 57, 1–11 (2015). https://doi.org/10.1007/s12033-014-9796-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-014-9796-3