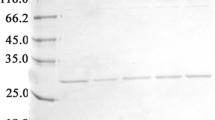
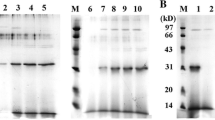
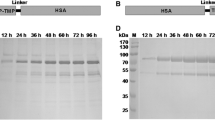
Abstract
Thrombolytic therapy by plasminogen activators (PAs) has been a main goal in the treatment of acute myocardial infarction. Despite improved outcomes of currently available thrombolytic therapies, all these agents have different drawbacks that may result in less than optimal outcomes. In order to make tissue plasminogen activator (tPA) more potent, while being more resistant to plasminogen activator inhibitor-1 (PAI-1) and having a higher affinity to fibrin, a new chimeric-truncated form of tPA (CT tPA) was designed and expressed in Pichia pastoris. This novel variant consists of a finger domain of Desmoteplase, an epidermal growth factor (EGF) domain, a kringle 1 (K1) domain, a kringle 2 (K2) domain, in which the lysine binding site (LBS) was deleted, and a protease domain, where the four amino acids lysine 296, arginine 298, arginine 299, and arginine 304 were substituted by aspartic acid. The chimera CT tPA showed 14-fold increase in its activity in the presence of fibrin compared to the absence of fibrin. Furthermore, CT tPA showed about 10-fold more potency than commercially available full-length tPA (Actylase®) and provided 1.2-fold greater affinity to fibrin. A residual activity of only 68 % was observed after incubation of Actylase® with PAI-1, however, 91 % activity remained for CT tPA. These promising findings suggest that the novel CT tPA variant might be an acceptable PA with superior characteristics and properties.





Similar content being viewed by others
References
Suarez, J. I. (2006). Acute myocardial infarction, ischemic stroke, sympathetic stress, and inflammation: Birds of a feather. Stroke, 37(10), 2449–2450.
Boersma, E., et al. (2003). Acute myocardial infarction. Lancet, 361(9360), 847–858.
Beaglehole, R., Saracci, R., & Panico, S. (2001). Cardiovascular diseases: Causes, surveillance and prevention. International Journal of Epidemiology, 30(Suppl 1), S1–S4.
Baruah, D. B., et al. (2006). Plasminogen activators: A comparison. Vascular Pharmacology, 44(1), 1–9.
Nordt, T. K., & Bode, C. (2003). Thrombolysis: Newer thrombolytic agents and their role in clinical medicine. Heart, 89(11), 1358–1362.
Jiao, J., Yu, M., & Ru, B. (2001). Characterization of a recombinant chimeric plasminogen activator with enhanced fibrin binding. Biochimica et Biophysica Acta, 1546(2), 399–405.
Liu, Y., et al. (2009). Construction of Pichia pastoris strain expressing salivary plasminogen activator from vampire bat (Desmodus rotundus). Sheng Wu Gong Cheng Xue Bao, 25(4), 566–574.
Liberatore, G. T., et al. (2003). Vampire bat salivary plasminogen activator (desmoteplase): A unique fibrinolytic enzyme that does not promote neurodegeneration. Stroke, 34(2), 537–543.
Patel, R., Ispoglou, S., & Apostolakis, S. (2014). Desmoteplase as a potential treatment for cerebral ischaemia. Expert Opinion on Investigational Drugs, 23(6), 865–873.
Piechowski-Jozwiak, B., & Bogousslavsky, J. (2013). The use of desmoteplase (bat saliva) in the treatment of ischaemia. Expert Opinion on Biological Therapy, 13(3), 447–453.
Paciaroni, M., Medeiros, E., & Bogousslavsky, J. (2009). Desmoteplase. Expert Opinion on Biological Therapy, 9(6), 773–778.
Majidzadeh, A. K., et al. (2010). Human tissue plasminogen activator expression in Escherichia coli using cytoplasmic and periplasmic cumulative power. Avicenna Journal of Medical Biotechnology, 2(3), 131–136.
Macauley-Patrick, S., et al. (2005). Heterologous protein production using the Pichia pastoris expression system. Yeast, 22(4), 249–270.
Cregg, J. M., et al. (2000). Recombinant protein expression in Pichia pastoris. Molecular Biotechnology, 16(1), 23–52.
Maccani, A., et al. (2014). Pichia pastoris secretes recombinant proteins less efficiently than Chinese hamster ovary cells but allows higher space-time yields for less complex proteins. Biotechnology Journal, 9(4), 526–537.
Felber, M., Pichler, H., & Ruth, C. (2014). Strains and molecular tools for recombinant protein production in Pichia pastoris. Methods in Molecular Biology, 1152, 87–111.
Mutch, N. J., et al. (2010). Polyphosphate modifies the fibrin network and down-regulates fibrinolysis by attenuating binding of tPA and plasminogen to fibrin. Blood, 115(19), 3980–3988.
Nieuwenhuizen, W. (2001). Fibrin-mediated plasminogen activation. Annals of the New York Academy of Sciences, 936, 237–246.
Hamilton, B. S., Brede, Y., & Tolbert, T. J. (2008). Expression and characterization of human glycosylated interleukin-1 receptor antagonist in Pichia pastoris. Protein Expression and Purification, 59(1), 64–68.
Guo, Y., et al. (2012). Purification and characterization of human IL-10/Fc fusion protein expressed in Pichia pastoris. Protein Expression and Purification, 83(2), 152–156.
Ku, H. K., et al. (2013). Interpretation of protein quantitation using the Bradford assay: comparison with two calculation models. Analytical Biochemistry, 434(1), 178–180.
Sadeghi, H. M., et al. (2012). Optimization of the expression of reteplase in Escherichia coli. Research in Pharmaceutical Sciences, 6(2), 87–92.
Nesheim, M., Fredenburgh, J. C., & Larsen, G. R. (1990). The dissociation constants and stoichiometries of the interactions of Lys-plasminogen and chloromethyl ketone derivatives of tissue plasminogen activator and the variant delta FEIX with intact fibrin. Journal of Biological Chemistry, 265(35), 21541–21548.
van Zonneveld, A. J., Veerman, H., & Pannekoek, H. (1986). On the interaction of the finger and the kringle-2 domain of tissue-type plasminogen activator with fibrin. Inhibition of kringle-2 binding to fibrin by epsilon-amino caproic acid. Journal of Biological Chemistry, 261(30), 14214–14218.
Davami, F., et al. (2011). A novel variant of t-PA resistant to plasminogen activator inhibitor-1; expression in CHO cells based on in silico experiments. BMB Reports, 44(1), 34–39.
Mahboudi, F., et al. (2013). A fed-batch based cultivation mode in Escherichia coli results in improved specific activity of a novel chimeric-truncated form of tissue plasminogen activator. Journal of Applied Microbiology, 114(2), 364–372.
George, D. J. G. (1989). Purified type I and type II t-PA. US Patent WO 89/09820.
Levine, G. N., Ali, M. N., & Schafer, A. I. (2001). Antithrombotic therapy in patients with acute coronary syndromes. Archives of Internal Medicine, 161(7), 937–948.
Dempfle, C. E., & Hennerici, M. G. (2011). Fibrinolytic treatment of acute ischemic stroke for patients on new oral anticoagulant drugs. Cerebrovascular Diseases, 32(6), 616–619.
Melandri, G., et al. (2009). Review of tenecteplase (TNKase) in the treatment of acute myocardial infarction. Vascular Health Risk and Management, 5(1), 249–256.
Millan, M., Dorado, L., & Davalos, A. (2010). Fibrinolytic therapy in acute stroke. Current Cardiology Reviews, 6(3), 218–226.
Bringmann, P., et al. (1995). Structural features mediating fibrin selectivity of vampire bat plasminogen activators. Journal of Biological Chemistry, 270(43), 25596–25603.
Madison, E. L., et al. (1990). Amino acid residues that affect interaction of tissue-type plasminogen activator with plasminogen activator inhibitor 1. Proceedings of the National Academy Science of the United States of America, 87(9), 3530–3533.
Furlan, A. J., et al. (2006). Dose escalation of desmoteplase for acute ischemic stroke (DEDAS): Evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke, 37(5), 1227–1231.
Wurm, F. M. (2004). Production of recombinant protein therapeutics in cultivated mammalian cells. Nature Biotechnology, 22(11), 1393–1398.
Walsh, G. (2006). Biopharmaceutical benchmarks 2006. Nature Biotechnology, 24(7), 769–776.
Krainer, F. W., et al. (2013). Knockout of an endogenous mannosyltransferase increases the homogeneity of glycoproteins produced in Pichia pastoris. Scientific Reports, 3, 3279.
Nagaoka, M. R., Kouyoumdjian, M., & Borges, D. R. (2003). Hepatic clearance of tissue-type plasminogen activator and plasma kallikrein in experimental liver fibrosis. Liver International, 23(6), 476–483.
Rouf, S. A., Moo-Young, M., & Chisti, Y. (1996). Tissue-type plasminogen activator: characteristics, applications and production technology. Biotechnology Advances, 14(3), 239–266.
Jalanko, A., et al. (1990). Production of human tissue-type plasminogen activator in different mammalian cell lines using an Epstein–Barr virus vector. Journal of Biotechnology, 15(1–2), 155–168.
Soleimani, M., et al. (2007). Expression of human tissue plasminogen activator in the trypanosomatid protozoan Leishmania tarentolae. Biotechnology and Applied Biochemistry, 48(Pt 1), 55–61.
Hua, Z. C., et al. (1994). Synthesis and expression of a gene from kringle-2 domain of tissue plasminogen activator in E. coli. Science in China Series B, 37(6), 667–676.
Weaver, W. D. (1996). The role of thrombolytic drugs in the management of myocardial infarction. Comparative clinical trials. European Heart Journal, 17(Suppl F), 9–15.
Davami, F., et al. (2010). Expression of a novel chimeric truncated t-PA in CHO cells based on in silico experiments. Journal of Biomedicine and Biotechnology, 2010, 108159.
Liu, Z., et al. (2012). Different expression systems for production of recombinant proteins in Saccharomyces cerevisiae. Biotechnology and Bioengineering, 109(5), 1259–1268.
Acknowledgments
The authors are deeply indebted to Dr. Oliver Spadiut (Vienna University of technology, Austria) for his insightful comments. This article is based on part of PhD dissertation and was fully supported by Pasteur Institute of Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saadatirad, A., Sardari, S., Kazemali, M. et al. Expression of a Novel Chimeric-Truncated tPA in Pichia pastoris with Improved Biochemical Properties. Mol Biotechnol 56, 1143–1150 (2014). https://doi.org/10.1007/s12033-014-9794-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-014-9794-5