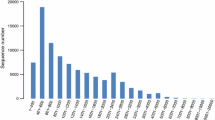
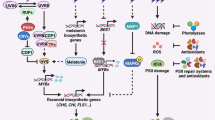
Abstract
UV-B radiation can damage biomolecules, such as DNA, RNA, and proteins, halting essential cellular processes; this damage is partly due to ROS generation. Plant secondary metabolites may protect against UV-B. Psychotria brachyceras Müll. Arg. (Rubiaceae), a subtropical shrub, produces brachycerine, a monoterpene indole alkaloid mainly accumulated in leaf tissues, which displays antioxidant and antimutagenic activities. Exposure of P. brachyceras cuttings to UV-B radiation significantly increases leaf brachycerine concentration. It has been suggested that this alkaloid might contribute to protection against UV-B damage both through its quenching activity on ROS and as UV shield. To identify differentially expressed genes of P. brachyceras in response to UV-B and investigate a possible influence of this stimulus on putative brachycerine-related genes, suppressive subtractive hybridization was applied. Complementary DNA from UV-B-treated leaves for 24 h was used as tester, and cDNA from untreated leaves, as driver. After BLASTX alignments, 134 sequences matched plant genes. Using quantitative RT-PCR, selected genes potentially related to brachycerine showed significant increases in transcription after UV-B exposure: tryptophan decarboxylase, ACC oxidase, UDP-glucose glucosyltransferase, lipase, and serine/threonine kinase. Results suggest a possible involvement of brachycerine in acute UV-B responses and show that alkaloid accumulation seems at least partly regulated at transcriptional level.

Similar content being viewed by others
References
Caldwell, M. M., Ballaré, C. L., Bornman, J. F., Flint, S. D., Björn, L. O., Teramura, A. H., et al. (2003). Terrestrial ecosystems, increased solar ultraviolet radiation and interactions with other climatic change factors. Photochemistry and Photobiology, 2, 29–38.
Caputo, C., Rutitzky, M., & Ballaré, C. L. (2006). Solar ultraviolet-B radiation alters the attractiveness of Arabidopsis plants to diamondback moths (Plutella xylostella L.): impacts on oviposition and involvement of the jasmonic acid pathway. Oecologia, 149, 81–90.
Jenkins, G. I. (2009). Signal transduction in responses to UV-B radiation. Annual Review of Plant Biology, 60, 407–431.
Frohnmeyer, H., & Staiger, D. (2003). Ultraviolet-B radiation-mediated responses in plants: balancing damage and protection. Plant Physiology, 133, 1420–1428.
Jansen, M. A. K., Gaba, V., & Greenberg, B. (1998). Higher plants and UV-B radiation: Balancing damage, repair and acclimation. Trends in Plant Sciences, 3, 131–135.
Ban, Y., Honda, C., Bessho, H., Pang, X., & Moriguchi, T. (2007). Suppression subtractive hybridization identifies genes induced in response to UV-B irradiation in apple skin: isolation of a putative UDP-glucose 4-epimerase. Journal of Experimental Botany, 58, 1825–1834.
Hollósy, F. (2002). Effects of ultraviolet radiation on plant cells. Micron, 33, 179–197.
Hahlbrock, K., & Scheel, D. (1989). Physiology and molecular biology of phenylpropanoid metabolism. Annual Review Plant Physiology and Plant Molecular Biology, 40, 347–369.
Schmitz-Hoerner, R., & Weissenböck, G. (2003). Contribution of phenolic compounds to the UV-B screening capacity of developing barley primary leaves in relation to DNA damage and repair under elevated UV-B levels. Phytochemistry, 64, 243–255.
Binder, B. Y., Peebles, C. A., Shanks, J. V., & San, K. Y. (2009). The effects of UV-B stress on the production of terpenoid indole alkaloids in Catharanthus roseus hairy roots. Biotechnology Progress, 25, 861–865.
Hartmann, T. (1991). Alkaloids. In G. A. Rosenthal & M. R. Berenbaum (Eds.), Herbivores: Their interaction with secondary plant metabolites (Vol. 1, pp. 79–121). San Diego: Academic Press.
Lydon, J., Casale, J. F., Kong, H., Sullivan, J. H., Daughtry, C. S., & Bailey, B. (2009). The effects of ambient solar UV radiation on alkaloid production by Erythroxylum novogranatense var. novogranatense. Photochemistry and Photobiology, 85, 1156–1161.
Hirata, K., Asada, M., Yatani, E., Miyamoto, K., & Miura, Y. (1993). Effects of near-ultraviolet light on alkaloid production in Catharanthus roseus plants. Planta Medica, 59, 46–50.
Hirata, K., Horiuchi, M., Asada, M., Ando, T., Miyamoto, K., & Miura, Y. (1992). Stimulation of dimeric alkaloid production by near-ultraviolet light in multiple shoot cultures of Catharanthus roseus. Journal of Fermentation and Bioengineering, 73, 222–225.
Ramani, S., & Chelliah, J. (2007). UV-B-induced signaling events leading to the enhanced-production of catharanthine in Catharanthus roseus cell suspension cultures. BMC Plant Biology, 7, 61.
Ouwerkerk, P. B., Hallard, D., Verpoorte, R., & Memelink, J. (1999). Identification of UV-B light-responsive regions in the promoter of the tryptophan decarboxylase gene from Catharanthus roseus. Plant Molecular Biology, 41, 491–503.
Green, R., & Fluhr, R. (1995). UV-B-induced PR-1 accumulation is mediated by active oxygen species. Plant Cell, 7, 203–212.
Mackerness, S., John, C. F., Jordan, B. R., & Thomas, B. (2001). Early signaling components in ultraviolet-B responses: distinct roles for different reactive oxygen species and nitric oxide. FEBS Letters, 489, 237–242.
Gregianini, T. S., Porto, D. D., do Nascimento, N. C., Fett, J. P., Henriques, A. T., & Fett-Neto, A. G. (2004). Environmental and ontogenetic control of accumulation of brachycerine, a bioactive indole alkaloid from Psychotria brachyceras. Journal of Chemical Ecology, 30, 2023–2036.
Kerber, V. A., Gregianini, T. S., Paranhos, J. T., Schwambach, J., Farias, F., Fett, J. P., et al. (2001). Brachycerine, a novel monoterpene indole alkaloid from Psychotria brachyceras. Journal Natural Products, 64, 677–679.
Gregianini, T. S., Silveira, V. C., Porto, D. D., Kerber, V. A., Henriques, A. T., & Fett-Neto, A. G. (2003). The alkaloid brachycerine is induced by ultraviolet radiation and is a singlet oxygen quencher. Photochemistry and Photobiology, 78, 470–474.
Nascimento, N. C., Fragoso, V., Moura, D. J., Silva, A. C. R., Fett-Neto, A. G., & Saffi, J. (2007). Antioxidant and antimutagenic effects of the crude foliar extract and the alkaloid brachycerine of Psychotria brachyceras. Environmental and Molecular Mutagenesis, 48, 728–734.
Diatchenko, L., Lau, Y. F., Campbell, A. P., Chenchik, A., Moqadam, F., Huang, B., et al. (1996). Suppression subtractive hybridization: a method for generating differentially regulated or tissue-specific cDNA probe and libraries. Proceedings of the National Academy of Sciences USA, 93, 6025–6030.
Caldwell, M. M. (1971). Solar UV irradiation and growth and development of higher plants. In A. C. Giese (Ed.), Photophysiology, Vol. 4. New York: Academic Press.
Barsalobres-Cavallari, C. F., Severino, F. E., Maluf, M. P., & Maia, I. G. (2009). Identification of suitable internal control genes for expression studies in Coffea arabica under different experimental conditions. BMC Molecular Biology, 10, 1.
Czechowski, T., Stitt, M., Altmann, T., Udvardi, M. K., & Scheible, W. (2005). Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiology, 139, 5–17.
Kim, B., Nam, H., Kim, S., & Chang, Y. (2003). Normalization of reverse transcription quantitative-PCR with housekeeping genes in rice. Biotechnology Letters, 25, 1869–1872.
Almeida, M. R., Ruedell, C. M., Ricachenevsky, F. K., Sperotto, R. A., Pasquali, G., & Fett-Neto, A. G. (2010). Reference gene selection for quantitative reverse transcription-polymerase chain reaction normalization during in vitro adventitious rooting in Eucalyptus globulus Labill. BMC Molecular Biology, 11, 1–12.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods, 25, 402–408.
Brosché, M., & Strid, A. (2003). Molecular events following perception of ultraviolet-B radiation by plants. Physiologia Plantarum, 117, 1–10.
Strid, A. (1993). Increased expression of defence genes in Pisum sativum after exposure to supplementary ultraviolet-B radiation. Plant Cell Physiology, 34, 949–953.
Willekens, H., Van Camp, W., Van Montagu, M., Inzé, D., Langebartels, C., & Sandermann, H., Jr. (1994). Ozone, sulfur dioxide, and ultraviolet B have similar effects on mRNA accumulation of antioxidant genes in Nicotiana plumbaginifolia L. Plant Physiology, 106, 1007–1014.
Demkura, P. V., Abdala, G., Baldwin, I. T., & Ballaré, C. L. (2010). Jasmonate-dependent and -independent pathways mediate specific effects of solar ultraviolet B radiation on leaf phenolics and antiherbivore defense. Plant Physiology, 152, 1084–1095.
Izaguirre, M., Mazza, C. A., Svatos, A., Baldwin, I. T., & Ballaré, C. L. (2007). Solar ultraviolet-B radiation and insect herbivory trigger partially overlapping phenolic responses in Nicotiana attenuata and Nicotiana longiflora. Annals of Botany, 99, 103–109.
Surplus, S. L., Jordan, B. R., Murphy, A. M., Carr, J. P., Thomas, B., & Mackerness, S. A.-H. (1998). Ultraviolet-B-induced responses in Arabidopsis thaliana: role of salicylic acid and reactive oxygen species in the regulation of transcripts encoding photosynthetic and acidic pathogenesis-related proteins. Plant, Cell and Environment, 21, 685–694.
Ries, G., Heller, W., Puchta, H., Sandermann, H., Seidlitz, H. K., & Hohn, B. (2000). Elevated UV-B radiation reduces genome stability in plants. Nature, 406, 98–101.
Sperotto, R. A., Ricachenevsky, F. K., Duarte, G. L., Boff, T., Lopes, K. L., Sperb, E. R., et al. (2009). Identification of up-regulated genes in flag leaves during rice grain filling and characterization of OsNAC5, a new ABA-dependent transcription factor. Planta, 230, 985–1002.
Li, D. M., Staehelin, C., Zhang, Y. S., & Peng, S. L. (2009). Identification of genes differentially expressed in Mikania micrantha during Cuscuta campestris infection by suppression subtractive hybridization. Journal of Plant Physiology, 166, 1423–1435.
Park, J. S., Choung, M. G., Kim, J. B., Hahn, B. S., Kim, J. B., Bae, S. C., et al. (2007). Genes up-regulated during red coloration in UV-B irradiated lettuce leaves. Plant Cell Reports, 26, 507–516.
Zinser, C., Seidlitz, H. K., Welzl, G., Sandermann, H., Heller, W., Ernst, D., et al. (2007). Transcriptional profiling of summer wheat, grown under different realistic UV-B irradiation regimes. Journal of Plant Physiology, 164, 913–922.
Gutierrez, L., Mauriat, M., Guénin, S., Pelloux, J., Lefebvre, J., Louvet, R., et al. (2008). The lack of a systematic validation of reference genes: a serious pitfall undervalued in reverse transcription-polymerase chain reaction (RT-PCR) analysis in plants. Plant Biotechnology Journal, 6, 609–618.
Hellemans, J., Mortier, G., De Paepe, A., Speleman, F., & Vandesompele, J. (2007). qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biology, 8, 19.
Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A., et al. (2002). Accurate normalisation of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology, 3, 1–11.
Andersen, C. L., Jensen, J. L., & Orntoft, T. F. (2004). Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Research, 64, 5245–5250.
Ramani, S., & Jayabaskaran, C. (2008). Enhanced catharanthine and vindoline production in suspension cultures of Catharanthus roseus by ultraviolet-B light. Journal of Molecular Signaling, 3, 9–14.
A-H-Mackerness, S., Surplus, S. L., Blake, P., John, C. F., Buchannan-Wollaston, V., Jordan, B. R., et al. (1999). Ultraviolet-B-induced stress and changes in gene expression in Arabidopsis thaliana: role of signalling pathways controlled by jasmonic acid, ethylene and reactive oxygen species. Plant, Cell and Environment, 22, 1413–1423.
Conconi, A., Smerdon, M. J., Howe, G. A., & Ryan, C. A. (1996). The octadecanoid signalling pathway in plants mediates a response to ultraviolet radiation. Nature, 383, 826–829.
Kende, H. (1993). Ethylene biosynthesis. Annual Review Plant Physiology and Plant Molecular Biology, 44, 283–307.
Roowi, S. H., Ho, C.-L., Alwee, S. S. R. S., Abdullah, M. O., & Napis, S. (2010). Isolation and characterization of differentially expressed transcripts from the suspension cells of oil palm (Elaeis guineensis Jacq.) in response to different concentration of auxins. Molecular Biotechnology, 46, 1–19.
Acknowledgments
This study was funded by the Brazilian Science Agencies CNPq (National Council for Scientific and Technological Development), CAPES (National Commission for Graduate Program Evaluation), and Fapergs (State Foundation for Research Support of Rio Grande do Sul). The authors thank Felipe K. Ricachenevsky, Plant Physiology Laboratory, Center for Biotechnology, Federal University of Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil, for helpful technical tips and discussions, and Shana de Souto Weber, Center for Biotechnology, UFRGS, Porto Alegre, RS, Brazil for providing bacterial competent cells.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
do Nascimento, N.C., Menguer, P.K., Sperotto, R.A. et al. Early Changes in Gene Expression Induced by Acute UV Exposure in Leaves of Psychotria brachyceras, a Bioactive Alkaloid Accumulating Plant. Mol Biotechnol 54, 79–91 (2013). https://doi.org/10.1007/s12033-012-9546-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-012-9546-3