Abstract
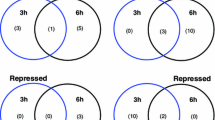
Salt stress is a mixture of ionic, osmotic, and oxidative stresses. The expression of TaSOS1 (a transmembrane Na+/H+ antiporter) and TaSOS4 [a cytoplasmic pyridoxal (PL) kinase] genes were measured in four different salinity levels and different time courses of salinity exposure using qRT-PCR technique. Mahuti (salt tolerant) and Alamut (salt sensitive) cultivars were used as cultivated wheat, and T. boeticum and Aegilops crassa as wild wheat plants. Salt-induced expression of TaSOS1 in these wild wheat plants indicates the presence of active TaSOS1 gene on the genomes A and D. The TaSOS1 and TaSOS4 transcript levels were found to be downregulated after salt treatment in all cultivars except in A. crassa, which was in contrast with its expression pattern in roots that was being upregulated from a very low-basal expression, after salt treatments. Duncan’s Multiple Range Test showed a significant difference between expression in the 200-mM NaCl concentration with the 50 and 100 mM for the TaSOS1 gene, and no significant difference for TaSOS4. Lack of significant correlation between the TaSOS1 and TaSOS4 gene expressions confirms the theory that PLP has no significant effect on the expression of the TaSOS1 gene in wheat leaves.


Similar content being viewed by others
References
Amthor, J. S., & McCree, K. J. (1990). Carbon balance of stressed plants: A conceptual model for integrating research results. In R. G. Ascher & J. R. Cumming (Eds.), Stress responses in plants: Adaptation and acclimation mechanisms (pp. 1–15). Wilmington, DE, USA: Wiley-Liss, Inc.
Apse, M. P., & Blumwald, E. (2002). Engineering salt tolerance in plants. Current Opinion in Biotechnology, 13, 146–150.
Barkla, B. J., & Pantoja, O. (1996). Physiology of ion transport across the tonoplast of higher plants. Annual Review of Plant Physiology and Plant Molecular Biology, 47, 127–157.
Benderradji, L., Brini, F., Amar, S. B., Kellou, K., Azaza, J., Masmoudi, K., et al. (2011). Sodium transport in the seedlings of two bread wheat (Triticum aestivum L.) genotypes showing contrasting salt stress tolerance. Australian Journal of Crop Science, 5(3), 233–241.
Blumwald, E., Aharon, G. S., & Apse, M. P. (2000). Sodium transport in plant cells. Biochimica et Biophysica Acta, 1465, 140–151.
Caldana, C., Scheible, W. R., Roeber, B. M., & Ruzicic, S. (2007). A quantitative RT-PCR platform for high-throughput expression profiling of 2500 rice transcription factors. Plant Methods, 3, 7.
Chen, Z. H., Pottosin, I. I., Cuin, T. A., et al. (2007). Root plasma membrane transporters controlling K+/Na+ homeostasis in saltstressed barley. Plant Physiology, 145, 1714–1725.
Cuin, T. A., Tian, Y., Betts, S. A., Chalmandrier, R., & Shabala, S. (2009). Ionic relations and osmotic adjustment in durum and bread wheat under saline conditions. Functional Plant Biology, 36, 1110–1119.
Cuin, T. A., Bose, J., Stefano, G., Jha, D., Tester, M., Mancuso, S., et al. (2011). Assessing the role of root plasma membrane and tonoplast Na+/H+ exchangers in salinity tolerance in wheat: In planta quantification methods. Plant, Cell and Environment, 34, 947–961. doi:10.1111/j.1365-3040.2011.02296.x.
Davenport, R., James, R. A., Zakrisson-Plogander, A., Tester, M., & Munns, R. (2005). Control of sodium transport in durum wheat. Plant Physiology, 137, 807–818.
Epstein, E. (1972). Mineral nutrition of plants: Principles and perspectives. New York: Wiley.
Gamarian, M., Malboobi, M., & Alizadeh, H. (2009). Expression analysis of salt stress induced genes in two salt-sensitive and –tolerant wheat cultivars. Genetic Novin, 4(1), 27–40.
Ganeshan, S., Vitamvas, P., Fowler, D. B., & Chibbar, R. N. (2008). Quantitative expression analysis of selected COR genes reveals their differential expression in leaf and crown tissues of wheat (Triticum aestivum L.) during an extended low temperature acclimation regimen. The Journal of Experimental Botany, 59, 2393–2402.
Ghavami, F., Malboobi, M. A., Ghannadha, M. R., Yazdi-Samadi, B., Mozaffari, J., & Jafar-Aghaei, M. (2004). Evaluation of salt tolerance of Iranian wheat cultivars at germination and seedling stages. Iranian Journal of Agricultural Sciences, 35(2), 453–464.
Ghavami, F., Malboobi, M. A., Ghannadha, M. R., Lohrasebi, T., Yazdi-Samadi, B., Mozaffari, J., et al. (2006). Up-regulation of succinate dehydrogenase and prophobilinogen deaminase in response to high salt concentration in wheat. Iranian Journal of Agricultural Sciences, 36(6), 1437–1444.
Hasegawa, P. M., Bressan, R. A., Zhu, J. K., & Bohnert, H. J. (2000). Plant cellular and molecular responses to high salinity. Annual Review of Plant Physiology and Plant Molecular Biology, 51, 463–499.
Hernandez, J. A., Olmos, E., Corpas, F. J., Sevilla, F., & Del-Rio, L. A. (1995). Salt-induced oxidative stress in chloroplasts of pea plants. Plant Science, 105, 151–167.
Horie, T., & Schroeder, J. I. (2004). Sodium transporters in plants diverse genes and physiological functions. Plant Physiology, 136, 2457–2462.
Horie, T., Hauser, F., & Schroeder, J. I. (2009). HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends in Plant Science, 14, 660–668.
Jain, M., Nijhawan, A., Tyagi, A. K., & Khurana, J. P. (2006). Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochemical and Biophysical Research, 345, 646–651.
Kamkar, B., Kafi, M., Nassiri-Mahallati, M. (2004). Determination of the most sensitive developmental period of wheat (Triticum aestivum) to salt stress to optimize saline water utilization. Australian Agronomy Conference. 2004, 12th AAC, 4th ICSC.
Kawasaki, S., Borchert, C., Deyholos, M., Wang, H., Brazille, S., Kawai, K., et al. (2001). Gene expression profiles during the initial phase of salt stress in rice. Plant Cell, 13, 889–905.
Kerepesi, I., & Galiba, G. (2000). Osmotic and salt stress-induced alteration in soluble carbohydrate content in wheat seedlings. Crop Science, 40, 482–487.
Larionov, A., Krause, A., & Miller, W. (2005). A standard curve based method for relative real time PCR data processing. BMC Bioinform, 6, 62. doi:10.1186/1471-2105-6-62.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods, 25, 402–408.
Martínez-Atíenza, J., Jiang, X. Y., Garciadeblás, B., Mendoza, I., Zhu, J.-K., Pardo, J. M., et al. (2007). Conservation of the salt overly sensitive pathway in rice. Plant Physiology, 143, 1001–1012.
Maughan, P. J., Turner, T. B., Coleman, C. E., Elzinga, D. B., Jellen, E. N., Morales, J. A., et al. (2009). Characterization of Salt Overly Sensitive 1 (SOS1) gene homoeologs in quinoa (Chenopodium quinoa Willd). Genome, 52(7), 647–657.
Munns, R., & Tester, M. (2008). Mechanisms of salinity tolerance. Annual Review of Plant Biology, 59, 651–681.
Munns, R. (2005). Genes and salt tolerance: Bringing them together. New Phytologist, 167, 645–663.
Nicot, N., Hausman, J. F., Hoffmann, L., & Evers, D. (2005). Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. The Journal of Experimental Botany, 56, 2907–2914.
Niu, X., Bressan, R. A., Hasegawa, P. M., & Pardo, J. M. (1995). Ion homeostasis in NaCl stress environments. Plant Physiology, 109, 735–742.
Ralevic, V., & Burnstock, G. (1998). Receptors for purines and pyrimidines. Pharmacological Reviews, 50, 413–492.
Sanders, D. (2000). Plant biology: The salty tale of Arabidopsis. Current Biology, 10, 486–488.
Shi, H., & Zhu, J. K. (2002). SOS 4 , a pyridoxal kinase gene, is required for root hair development in Arabidopsis. Plant Physiology, 129, 585–593.
Shi, H., Lining, X., Becky, S., Tiegang, L., & Zhu, J. K. (2002). The Arabidopsis salt overly sensitive 4 mutants uncover a critical role for vitamin B6 in plant salt tolerance. The Plant Cell, 14, 575–588.
Shi, H., Ishitani, M., Kim, C., & Zhu, J. K. (2000). The Arabidopsis thaliana salt tolerance gene SOS 1 encodes a putative Na+/H+ antiporter. Proceedings of the National Academy of Sciences of the United States of America, 97, 6896–6901.
Subbarao, G. V., & Johansen, C. (2003). Strategies and scope for improving salinity tolerance in crop plants. In M. Pessaraki (Ed.), Hand book of plant and crop stress (pp. 1069–1087). New York: Marcel Dekker Inc.
Wagdy, A. S., & Ali, H. H. (2002). Generation of transgenic wheat plants producing high levels of the osmoprotectant proline. Biotechnology Letters, 24, 721–725.
Xu, H. X., Jiang, X. Y., Zhan, K. H., Cheng, X. Y., Chen, X. J., Pardo, J. M., et al. (2008). Functional characterization of a wheat plasma membrane Na+/H+ antiporter in yeast. Archives of Biochemistry and Biophysics, 473, 8–15.
Yokoi, S., Bressan, R. A., & Hasegawa, P. M. (2002). Salt stress tolerance of plants. JIRCAS Working Report, pp. 25–33.
Zhu, J. K. (2000). Genetic analysis of plant salt tolerance using Arabidopsis. Plant Physiology, 124, 941–948.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramezani, A., Niazi, A., Abolimoghadam, A.A. et al. Quantitative Expression Analysis of TaSOS1 and TaSOS4 Genes in Cultivated and Wild Wheat Plants Under Salt Stress. Mol Biotechnol 53, 189–197 (2013). https://doi.org/10.1007/s12033-012-9513-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-012-9513-z