Abstract
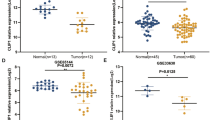
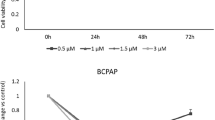
Papillary thyroid carcinoma (PTC) is a common endocrine malignancy. The pathology of PTC is far from clear. As a kinase that can be targeted, the role of TNIK in PTC has not been investigated. This study was focused on the effects and molecular mechanisms of TNIK in PTC. Both public datasets and clinical specimens were used to verify TNIK expression. The effects of TNIK were investigated in both cell lines and mice models. Transcriptome analysis was used to explore the underlying mechanism of TNIK. Immunofluorescence, wound healing, and qRT-PCR assays were used to validate the mechanism of TNIK in PTC. The therapeutic effects of TNIK inhibitor NCB-0846 were evaluated by flow cytometry, western blot, and subcutaneous xenografts mice. TNIK expression was upregulated in PTC tissues. TNIK knockdown could suppress cell proliferation and tumor growth in no matter cell models or nude mice. The transcriptome analysis, GO enrichment analysis, and GSEA analysis results indicated TNIK was highly correlated with cytoskeleton, cell motility, and Wnt pathways. The mechanistic studies demonstrated that TNIK regulated cytoskeleton remodeling and promoted cell migration. NCB-0846 significantly inhibited TNIK kinase activity, induced cell apoptosis, and activated apoptosis-related proteins in a dose-dependent manner. In addition, NCB-0846 inhibited tumor growth in tumor-bearing mice. In summary, we proposed a novel regulatory mechanism in which TNIK-mediated cytoskeleton remodeling and cell migration to regulate tumor progression in PTC. TNIK is a therapeutic target in PTC and NCB-0846 would act as a novel targeted drug for PTC therapy.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Yang YF, Yu B, Zhang XX, et al. Identification of TNIK as a novel potential drug target in thyroid cancer based on protein druggability prediction. Medicine. 2021;100(16):e25541.
Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295(18):2164–7.
Du LB, Li HZ, Wang XH, et al. Analysis of cancer incidence in Zhejiang cancer registry in China during 2000 to 2009. Asian Pac J Cancer Prev. 2014;15(14):5839–43.
Xie WC, Chan MH, Mak KC, et al. Trends in the incidence of 15 common cancers in Hong Kong, 1983–2008. Asian Pac J Cancer Prev. 2012;13(8):3911–6.
Xie SH, Chen J, Zhang B, et al. Time trends and age-period-cohort analyses on incidence rates of thyroid cancer in Shanghai and Hong Kong. BMC Cancer. 2014;14:975.
Li Z, Lin W, Zheng J, et al. Identification of immune-related lncRNAs to improve the prognosis prediction for patients with papillary thyroid cancer. Biosci Rep. 2021;41(2):BSR20204086.
Yuan R, Li Y, Fu Y, et al. TNIK influence the effects of antipsychotics on Wnt/beta-catenin signaling pathway. Psychopharmacology. 2021;238(11):3283–92.
Yu DH, Zhang X, Wang H, et al. The essential role of TNIK gene amplification in gastric cancer growth. Oncogenesis. 2014;2: e89.
Masuda M, Uno Y, Ohbayashi N, et al. TNIK inhibition abrogates colorectal cancer stemness. Nat Commun. 2016;7:12586.
Lee RS, Zhang L, Berger A, et al. Characterization of the ERG-regulated Kinome in Prostate Cancer Identifies TNIK as a Potential Therapeutic Target. Neoplasia. 2019;21(4):389–400.
Liu Y, Gao S, Jin Y, et al. Bioinformatics analysis to screen key genes in papillary thyroid carcinoma. Oncol Lett. 2020;19(1):195–204.
Ni X, Zhang J. Pediatric otolaryngology-head and neck surgery in China: present situation and future prospects. Pediatr Investig. 2019;3(3):137–40.
Cabanillas ME, Ryder M, Jimenez C. Targeted therapy for advanced thyroid cancer: kinase inhibitors and beyond. Endocr Rev. 2019;40(6):1573–604.
Masuda M, Sawa M, Yamada T. Therapeutic targets in the Wnt signaling pathway: feasibility of targeting TNIK in colorectal cancer. Pharmacol Ther. 2015;156:1–9.
Torres-Ayuso P, An E, Nyswaner KM, et al. TNIK is a therapeutic target in lung squamous cell carcinoma and regulates FAK activation through merlin. Cancer Discov. 2021;11(6):1411–23.
Raposo L, Morais S, Oliveira MJ, et al. Trends in thyroid cancer incidence and mortality in Portugal. Eur J Cancer Prev. 2017;26(2):135–43.
Al-Salamah SM, Khalid K, Bismar HA. Incidence of differentiated cancer in nodular goiter. Saudi Med J. 2002;23(8):947–52.
Nikiforova MN, Tseng GC, Steward D, et al. MicroRNA expression profiling of thyroid tumors: biological significance and diagnostic utility. J Clin Endocrinol Metab. 2008;93(5):1600–8.
Stoupa A, Kariyawasam D, Polak M, et al. Genetics of congenital hypothyroidism: modern concepts. Pediatr Investig. 2022;6(2):123–34.
Bunone G, Vigneri P, Mariani L, et al. Expression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical pathological features. Am J Pathol. 1999;155(6):1967–76.
Schouest KR, Kurasawa Y, Furuta T, et al. The germinal center kinase GCK-1 is a negative regulator of MAP kinase activation and apoptosis in the C. elegans germline. PLoS ONE. 2009;4(10):e7450.
Oehrl W, Kardinal C, Ruf S, et al. The germinal center kinase (GCK)-related protein kinases HPK1 and KHS are candidates for highly selective signal transducers of Crk family adapter proteins. Oncogene. 1998;17(15):1893–901.
Yu DH, Zhang X, Wang H, et al. The essential role of TNIK gene amplification in gastric cancer growth. Oncogenesis. 2014;2(2): e89.
Sato K, Padgaonkar AA, Baker SJ, et al. Simultaneous CK2/TNIK/DYRK1 inhibition by 108600 suppresses triple negative breast cancer stem cells and chemotherapy-resistant disease. Nat Commun. 2021;12(1):4671.
Mahmoudi T, Li VS, Ng SS, et al. The kinase TNIK is an essential activator of Wnt target genes. EMBO J. 2009;28(21):3329–40.
Jho EH, Zhang T, Domon C, et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22(4):1172–83.
McGarry DJ, Armstrong G, Castino G, et al. MICAL1 regulates actin cytoskeleton organization, directional cell migration and the growth of human breast cancer cells as orthotopic xenograft tumours. Cancer Lett. 2021;519:226–36.
Jeong YJ, Hwang SK, Magae J, et al. Ascofuranone suppresses invasion and F-actin cytoskeleton organization in cancer cells by inhibiting the mTOR complex 1 signaling pathway. Cell Oncol. 2020;43(5):793–805.
Ohishi T, Yoshida H, Katori M, et al. Tankyrase-binding protein TNKS1BP1 regulates actin cytoskeleton rearrangement and cancer cell invasion. Cancer Res. 2017;77(9):2328–38.
Zhang P, Teng J, Wang L. Multiwalled carbon nanotubes inhibit cell migration and invasion by destroying actin cytoskeleton via mitochondrial dysfunction in ovarian cancer cells. Aging. 2020;12(24):25294–303.
Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326(5957):1208–12.
Akhshi TK, Wernike D, Piekny A. Microtubules and actin crosstalk in cell migration and division. Cytoskeleton. 2014;71(1):1–23.
Hall A. The cytoskeleton and cancer. Cancer Metastasis Rev. 2009;28(1–2):5–14.
Chinchole A, Lone KA, Tyagi S. MLL regulates the actin cytoskeleton and cell migration by stabilising Rho GTPases via the expression of RhoGDI1. J Cell Sci. 2022;135(20):jcs260042.
Best M, Gale ME, Wells CM. PAK-dependent regulation of actin dynamics in breast cancer cells. Int J Biochem Cell Biol. 2022;146:106207.
Grandy C, Port F, Pfeil J, et al. Influence of ROCK pathway manipulation on the actin cytoskeleton height. Cells. 2022;11(3):430.
Wang Z, Sun L, Liang S, et al. GPER stabilizes F-actin cytoskeleton and activates TAZ via PLCbeta-PKC and Rho/ROCK-LIMK-Cofilin pathway. Biochem Biophys Res Commun. 2019;516(3):976–82.
Ma X, Dang Y, Shao X, et al. Ubiquitination and long non-coding RNAs regulate actin cytoskeleton regulators in cancer progression. Int J Mol Sci. 2019;20(12):2997.
Ancker OV, Kruger M, Wehland M, et al. Multikinase inhibitor treatment in thyroid cancer. Int J Mol Sci. 2019;21(1):10.
Meng X, Wang H, Zhao J, et al. Apatinib inhibits cell proliferation and induces autophagy in human papillary thyroid carcinoma via the PI3K/Akt/mTOR signaling pathway. Front Oncol. 2020;10:217.
Jeong JH, Oh JM, Jeong SY, et al. Combination treatment with the BRAF(V600E) inhibitor vemurafenib and the BH3 mimetic navitoclax for BRAF-mutant thyroid carcinoma. Thyroid. 2019;29(4):540–8.
Chattopadhyay C, El-Naggar AK, Williams MD, et al. Small molecule c-MET inhibitor PHA665752: effect on cell growth and motility in papillary thyroid carcinoma. Head Neck. 2008;30(8):991–1000.
Funding
This work was supported by the Beijing-Tianjin-Hebei Integration Project (No. J200004, 20JCZXJC00120), Beijing Research Ward Demonstration Unit (BCRW202101), Beijing municipal science & technology commission (No. Z201100005520077), and Beijing Hospitals Authority Innovation Studio of Young Staff Funding Support (No. 202126).
Author information
Authors and Affiliations
Contributions
XN and YLG designed the experiment and revised the paper; RQZ and YBY performed most of the experiments and wrote the draft; YRY and MZ contributed to bioinformatic analysis; SCW and JLL performed tumor tissue collection and immunohistochemistry detection; XZ, YC, and LFH cultured the cells; XQZ and RLZ conducted the statistical analysis. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Ethical approval
The Ethics Committees of Beijing Children’s Hospital approved this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, R., Yu, Y., Yang, Y. et al. Therapeutic targeting of TNIK in papillary thyroid carcinoma: a novel approach for tumor growth suppression. Med Oncol 41, 160 (2024). https://doi.org/10.1007/s12032-024-02380-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-024-02380-y