Abstract
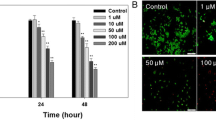
We previously reported that chitosan nanoparticle-encapsulated Naringenin (CS-NPs/NAR) could scavenge free radicals at lower doses and be cytotoxic to cancer cells. The current study continues to focus on the mechanism behind CS-NPs/NAR-induced breast cancer cell (MDA-MB-231) death. MDA-MB-231 cells were treated with higher concentrations (100, 200, and 200 µg) of Chitosan nanoparticles (CS-NPs), naringenin (NAR), and chitosan-encapsulated naringenin (CS-NPs/NAR). The cell viability, proliferation, and oxidative stress parameters, such as nitric oxide [NO], xanthine oxidase (XOD), and xanthine dehydrogenase (XDH) levels, were analyzed. ROS levels were determined through DCFDA analysis. MTT-based cell cytotoxicity and BrdU cell proliferation analysis depicted the cytotoxicity effects (37% and 29% for 24 and 48 h) and exhibited a reduction in the proliferation of MDA-MB-231 by CS-NPs/NAR. A significant increase in NO content, XOD, a decrease in XDH, and an increase in ROS levels were observed upon treatment with CS-NPs/NAR. Fluorescent images suggested the increase in the ROS level upon treatment with CS-NPs/NAR in cancer cells, and the results suggested that it could induce apoptosis. Further, to confirm this, the activity of caspase-3 was analyzed through western blotting, and the result suggested that the higher concentration of CS-NPs/NAR has increased the activation of procaspase3 when compared to free NAR. Hence, the current investigation concludes that high doses of CS-NPs/NAR induce and increase oxidative stress and so increased activation of procaspase3 may lead to cancer cell apoptosis and reduction in cell proliferation.








Similar content being viewed by others
Data availability
Not applicable.
References
Anitha A, Sowmya S, Kumar PTS, Deepthi S, Chennazhi KP, Ehrlich H, Tsurkan M, Jayakumar R. Chitin and chitosan in selected biomedical applications. Prog Polym Sci. 2014. https://doi.org/10.1016/j.progpolymsci.2014.02.008.
Trachootham D, Lu W, Ogasawara MA, Nilsa RDV, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10:1343–74. https://doi.org/10.1089/ars.2007.1957.
Kumar SP, Birundha K, Kaveri K, Devi KTR. Antioxidant studies of chitosan nanoparticles containing naringenin and their cytotoxicity effects in lung cancer cells. Int J Biol Macromol. 2015;78:87–95. https://doi.org/10.1016/j.ijbiomac.2015.03.045.
Jayaraman J, Jesudoss VAS, Menon VP, Namasivayam N. Anti-inflammatory role of naringenin in rats with ethanol induced liver injury. Toxicol Mech Methods. 2012;22:568–76. https://doi.org/10.3109/15376516.2012.707255.
McCord JM, Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968;243:5753–60. https://doi.org/10.1016/S0021-9258(18)91929-0.
Gokulnath M, Partridge NC, Selvamurugan N. Runx2, a target gene for activating transcription factor-3 in human breast cancer cells. Tumor Biol. 2015. https://doi.org/10.1007/s13277-014-2796-x.
Perillo B, Di Donato M, Pezone A, Di Zazzo E, Giovannelli P, Galasso G, Castoria G, Migliaccio A. ROS in cancer therapy: the bright side of the moon. Exp Mol Med. 2020. https://doi.org/10.1038/s12276-020-0384-2.
Huang X, Shi X, Zhou J, Li S, Zhang L, Zhao H, Kuang X, Li J. The activation of antioxidant and apoptosis pathways involved in damage of human proximal tubule epithelial cells by PM2.5 exposure. Sci Eur Environ. 2020. https://doi.org/10.1186/s12302-019-0284-z.
Bastianetto S, Ramassamy C, Poirier J, Quirion R. Dehydroepiandrosterone (DHEA) protects hippocampal cells from oxidative stress-induced damage. Brain Res Mol Brain Res. 1999;66:35–41. https://doi.org/10.1016/S0169-328X(99)00002-9.
Halliwell B, Gutteridge JM. Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett. 1981;128:347–52. https://doi.org/10.1016/0014-5793(81)80114-7.
Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–51. https://doi.org/10.1016/j.redox.2015.08.020.
Ramya Devi KT, Sivalingam N. Cichorium intybus attenuates Streptozotocin-induced pancreatic β-cell damage by inhibiting NF-κB activation and oxidative stress. J Appl Biomed. 2020;18:70–9. https://doi.org/10.32725/jab.2020.010.
Fathi M, Abdolahinia ED, Barar J, Omidi Y. Smart stimuli-responsive biopolymeric nanomedicines for targeted therapy of solid tumors. Nanomedicine. 2020;15:2171–200. https://doi.org/10.2217/nnm-2020-0146.
Bashir SM, Ahmed Rather G, Patrício A, Haq Z, Sheikh AA, Ul Shah MZH, Singh H, Khan AA, Imtiyaz S, Ahmad SB, et al. Chitosan nanoparticles: a versatile platform for biomedical applications. Materials. 2022;15:6521. https://doi.org/10.3390/ma15196521.
Luque-Bolivar A, Pérez-Mora E, Villegas VE, Rondón-Lagos M. Resistance and overcoming resistance in breast cancer. Breast Cancer Targets Ther. 2020;12:211–29. https://doi.org/10.2147/BCTT.S270799.
Frigaard J, Jensen JL, Galtung HK, Hiorth M. The potential of chitosan in nanomedicine: an overview of the cytotoxicity of chitosan based nanoparticles. Front Pharmacol. 2022;13:1492. https://doi.org/10.3389/fphar.2022.880377.
Chipuk JE, Bouchier-Hayes L, Green DR. Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ. 2006. https://doi.org/10.1038/sj.cdd.4401963.
Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med. 2003;348:1365–75. https://doi.org/10.1056/NEJMra022366.
Rahmanian N, Hosseinimehr SJ, Khalaj A. The paradox role of caspase cascade in ionizing radiation therapy. J Biomed Sci. 2016;23:88. https://doi.org/10.1186/s12929-016-0306-8.
McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5:1–28. https://doi.org/10.1101/cshperspect.a008656.
Arslan H, Özdemir S, Altun S. Cypermethrin toxication leads to histopathological lesions and induces inflammation and apoptosis in common carp (Cyprinus carpio L). Chemosphere. 2017;180:491–9. https://doi.org/10.1016/j.chemosphere.2017.04.057.
Porter A, Janicke R. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. https://doi.org/10.1038/sj.cdd.4400476.
Ponder KG, Boise LH. The prodomain of caspase-3 regulates its own removal and caspase activation. Cell Death Discov. 2019;5:56. https://doi.org/10.1038/s41420-019-0142-1.
Madesh M, Balasubramanian KA. A microtiter plate assay for superoxide using MTT reduction method. Indian J Biochem Biophys. 1997;34:535–9 (PMID: 9803669).
Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9. https://doi.org/10.1073/pnas.84.24.9265.
Parks DA, Granger DN. Ischemia-reperfusion injury: a radical view. Hepatology. 1988;8:680–2. https://doi.org/10.1002/hep.1840080341.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. https://doi.org/10.1016/0003-2697(76)90527-3.
Eruslanov E, Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol Biol. 2010;594:57–72. https://doi.org/10.1007/978-1-60761-411-1_4.
Datta S, Choudhury D, Das A, Das Mukherjee D, Das N, Roy SS, Chakrabarti G. Paclitaxel resistance development is associated with biphasic changes in reactive oxygen species, mitochondrial membrane potential and autophagy with elevated energy production capacity in lung cancer cells: a chronological study. Tumor Biol. 2017. https://doi.org/10.1177/1010428317694314.
Agarwal A, Kasinathan A, Ganesan R, Balasubramanian A, Bhaskaran J, Suresh S, Srinivasan R, Aravind KB, Sivalingam N. Curcumin induces apoptosis and cell cycle arrest via the activation of reactive oxygen species-independent mitochondrial apoptotic pathway in Smad4 and p53 mutated colon adenocarcinoma HT29 cells. Nutr Res. 2018;51:67–81. https://doi.org/10.1016/j.nutres.2017.12.011.
Crowley LC, Scott AP, Marfell BJ, Boughaba JA, Chojnowski G, Waterhouse NJ. Measuring cell death by propidium iodide uptake and flow cytometry. Cold Spring Harb Protoc. 2016. https://doi.org/10.1101/pdb.prot087163.
Ahmadian S, Barar J, Saei AA, Fakhree MAA, Omidi Y. Cellular toxicity of nanogenomedicine in MCF-7 cell line: MTT assay. J Vis Exp JoVE. 2009. https://doi.org/10.3791/1191.
Ahamed M, Ali D, Alhadlaq HA, Akhtar MJ. Nickel oxide nanoparticles exert cytotoxicity via oxidative stress and induce apoptotic response in human liver cells (HepG2). Chemosphere. 2013;93:2514–22. https://doi.org/10.1016/j.chemosphere.2013.09.047.
Sridevi DV, Ramya Devi KT, Jayakumar N, Sundaravadivel E. PH dependent synthesis of TiO2nanoparticles exerts its effect on bacterial growth inhibition and osteoblasts proliferation. AIPAdv. 2020. https://doi.org/10.1063/50020029.
Friederich M, Hansell P, Palm F. Diabetes, oxidative stress, nitric oxide and mitochondria function. Curr Diabetes Rev. 2009;5:120–44. https://doi.org/10.2174/157339909788166800.
Ramya Devi KT. Cichorium intybus L. accords hepatoprotection in Streptozotocin induced diabetes mellitus in Wistar rats. Res J Biotechnol. 2018;13:42–5.
Tawa P, Hell K, Giroux A, Grimm E, Han Y, Nicholson DW, Xanthoudakis S. Catalytic activity of caspase-3 is required for its degradation: stabilization of the active complex by synthetic inhibitors. Cell Death Differ. 2004;11:439–47. https://doi.org/10.1038/sj.cdd.4401360.
Bekele R, Venkatraman G, Liu RZ, et al. Oxidative stress contributes to the tamoxifen-induced killing of breast cancer cells: implications for tamoxifen therapy and resistance. Sci Rep. 2016;6:21164. https://doi.org/10.1038/srep21164.
Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer lett. 2008;269(2):315–25. https://doi.org/10.1016/j.canlet.2008.03.046.
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
KTR and MKJ: designed this work. MRG has done the work based on FACS and confocal images, and the technical help had been taken from KD for fluorescent imaging. The manuscript was written by KTR and reviewed by KTR and MKJ.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramya Devi, K.T., Jaganathan, M.K., Ganesh, M.R. et al. Chitosan-encapsulated naringenin promotes ROS mediated through the activation of executioner caspase-3. Med Oncol 41, 3 (2024). https://doi.org/10.1007/s12032-023-02227-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02227-y