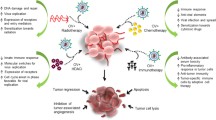
Abstract
Viruses are being researched as cutting-edge therapeutic agents in cancer due to their selective oncolytic action against malignancies. Immuno-oncolytic viruses are a potential category of anticancer treatments because they have natural features that allow viruses to efficiently infect, replicate, and destroy cancer cells. Oncolytic viruses may be genetically modified; engineers can use them as a platform to develop additional therapy modalities that overcome the limitations of current treatment approaches. In recent years, researchers have made great strides in the understanding relationship between cancer and the immune system. An increasing corpus of research is functioning on the immunomodulatory functions of oncolytic virus (OVs). Several clinical studies are currently underway to determine the efficacy of these immuno-oncolytic viruses. These studies are exploring the design of these platforms to elicit the desired immune response and to supplement the available immunotherapeutic modalities to render immune-resistant malignancies amenable to treatment. This review will discuss current research and clinical developments on Vaxinia immuno-oncolytic virus.



Similar content being viewed by others
Data availability
This submission does not require any availability of data and materials as this is a review paper.
References
Cancer in 2022—CPR22. (n.d.). Cancer Progress Report., https://cancerprogressreport.aacr.org/progress/cpr22-contents/cpr22-cancer-in-2022/. Accessed 30 April 2023
Cancer. (n.d.)., https://www.who.int/news-room/fact-sheets/detail/cancer. Accessed 23 Aug 2022
Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149(4):778–89. https://doi.org/10.1002/ijc.33588.
Lauer UM, Beil J. Oncolytic viruses: challenges and considerations in an evolving clinical landscape. Future Oncol. 2022;18(24):2713–32. https://doi.org/10.2217/fon-2022-0440.
Kayode AA, Eya IE, Kayode OT. A short review on cancer therapeutics. Phys Sci Rev. 2022. https://doi.org/10.1515/psr-2021-0169.
Nenclares P, Harrington KJ. The biology of cancer. Medicine. 2020;48(2):67–72. https://doi.org/10.1016/j.mpmed.2019.11.001.
Cattley RC, Radinsky BR. Cancer therapeutics: understanding the mechanism of action. Toxicol Pathol. 2004;32(1_suppl):116–21. https://doi.org/10.1080/01926230490426507.
Vesely MD, Schreiber RD. Cancer immunoediting: Antigens, mechanisms, and implications to cancer immunotherapy: tumor antigens and cancer immunoediting. Ann N Y Acad Sci. 2013;1284(1):1–5. https://doi.org/10.1111/nyas.12105.
Gubin MM, Vesely MD. Cancer immunoediting in the era of immuno-oncology. Clin Cancer Res. 2022. https://doi.org/10.1158/1078-0432.CCR-21-1804.
Wilczyński JR, Nowak M. Cancer Immunoediting Elimination, Equilibrium, and Immune Escape in Solid Tumors. In: Klink M, Szulc-Kielbik I, editors. Interaction of Immune and Cancer Cells, vol. 113. Cham: Springer International Publishing; 2022. p. 1–57. https://doi.org/10.1007/978-3-030-91311-3_1.
Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, Kolahian S, Javaheri T, Zare P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. 2020;18(1):59. https://doi.org/10.1186/s12964-020-0530-4.
Atsou K, Khou S, Anjuère F, Braud VM, Goudon T. Analysis of the equilibrium phase in immune-controlled tumors provides hints for designing better strategies for cancer treatment. Front Oncol. 2022;12:878827. https://doi.org/10.3389/fonc.2022.878827.
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: From immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–8. https://doi.org/10.1038/ni1102-991.
Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–48. https://doi.org/10.1016/j.immuni.2004.07.017.
Whiteside T. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol. 2006;16(1):3–15. https://doi.org/10.1016/j.semcancer.2005.07.008.
Printezi MI, Kilgallen AB, Bond MJG, Štibler U, Putker M, Teske AJ, Cramer MJ, Punt CJA, Sluijter JPG, Huitema ADR, May AM, van Laake LW. Toxicity and efficacy of chronomodulated chemotherapy: a systematic review. Lancet Oncol. 2022;23(3):e129–43. https://doi.org/10.1016/S1470-2045(21)00639-2.
Alfarouk KO, Stock C-M, Taylor S, Walsh M, Muddathir AK, Verduzco D, Bashir AHH, Mohammed OY, Elhassan GO, Harguindey S, Reshkin SJ, Ibrahim ME, Rauch C. Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int. 2015;15(1):71. https://doi.org/10.1186/s12935-015-0221-1.
Garmaroudi GA, Karimi F, Naeini LG, Kokabian P, Givtaj N. Therapeutic Efficacy of oncolytic viruses in fighting cancer: recent advances and perspective. Oxid Med Cell Longev. 2022;2022:1–14. https://doi.org/10.1155/2022/3142306.
Bommareddy PK, Shettigar M, Kaufman HL. Integrating oncolytic viruses in combination cancer immunotherapy. Nat Rev Immunol. 2018;18(8):498–513. https://doi.org/10.1038/s41577-018-0014-6.
de Graaf JF, de Vor L, Fouchier RAM, van den Hoogen BG. Armed oncolytic viruses: a kick-start for anti-tumor immunity. Cytokine Growth Factor Rev. 2018;41:28–39. https://doi.org/10.1016/j.cytogfr.2018.03.006.
Yuan Y, Zhang J, Kessler J, Rand J, Modi B, Chaurasiya S, Murga M, Tang A, Martinez N, Meisen H, Yamauchi D, Yost SE, Chong LMO, Seiz A, Nixon B, Ede N, Waisman JR, Stewart DB, Mortimer JE, Fong Y. Phase I study of intratumoral administration of CF33-HNIS-antiPDL1 in patients with metastatic triple negative breast cancer. J Clin Oncol. 2022;40(16_suppl):e13070–e13070. https://doi.org/10.1200/JCO.2022.40.16_suppl.e13070.
Imugene (ASX: IMU). (n.d.). https://www.imugene.com/. Accessed 23 Aug 2022
Greseth MD, Traktman P. The life cycle of the vaccinia virus genome. Ann Rev Virol. 2022. https://doi.org/10.1146/annurev-virology-091919-104752.
Zhang Z, Dong L, Zhao C, Zheng P, Zhang X, Xu J. Vaccinia virus-based vector against infectious diseases and tumors. Hum Vaccin Immunother. 2021;17(6):1578–85. https://doi.org/10.1080/21645515.2020.1840887.
Mackett M, Smith GL, Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci. 1982;79(23):7415–9. https://doi.org/10.1073/pnas.79.23.7415.
Woo Y, Zhang Z, Yang A, Chaurasiya S, Park AK, Lu J, Kim S-I, Warner SG, Von Hoff D, Fong Y. Novel chimeric immuno-oncolytic virus CF33-hNIS-antiPDL1 for the treatment of pancreatic cancer. J Am Coll Surg. 2020;230(4):709–17. https://doi.org/10.1016/j.jamcollsurg.2019.12.027.
Mojic M, Takeda K, Hayakawa Y. The dark side of IFN-γ: its role in promoting cancer immunoevasion. Int J Mol Sci. 2017;19(1):89. https://doi.org/10.3390/ijms19010089.
Schirrmacher V. Molecular Mechanisms of anti-neoplastic and immune stimulatory properties of oncolytic newcastle disease virus. Biomedicines. 2022;10(3):562. https://doi.org/10.3390/biomedicines10030562.
Esfahani K, Roudaia L, Buhlaiga N, Del Rincon SV, Papneja N, Miller WH. A Review of cancer immunotherapy: from the past, to the present, to the future. Curr Oncol. 2020;27(12):87–97. https://doi.org/10.3747/co.27.5223.
Davola ME, Mossman KL. Oncolytic viruses: how “lytic” must they be for therapeutic efficacy. OncoImmunology. 2019;8(6):e1581528. https://doi.org/10.1080/2162402X.2019.1596006.
Rojas-Domínguez A, Arroyo-Duarte R, Rincón-Vieyra F, Alvarado-Mentado M. Modeling cancer immunoediting in tumor microenvironment with system characterization through the ising-model Hamiltonian. BMC Bioinform. 2022;23(1):200. https://doi.org/10.1186/s12859-022-04731-w.
Nirmal AJ, Maliga Z, Vallius T, Quattrochi B, Chen AA, Jacobson CA, Pelletier RJ, Yapp C, Arias-Camison R, Chen Y-A, Lian CG, Murphy GF, Santagata S, Sorger PK. The Spatial landscape of progression and immunoediting in primary melanoma at single-cell resolution. Cancer Discov. 2022;12(6):1518–41. https://doi.org/10.1158/2159-8290.CD-21-1357.
Christie JD, Chiocca EA. Treat and repeat: oncolytic virus therapy for brain cancer. Nat Med. 2022;28(8):1540–2. https://doi.org/10.1038/s41591-022-01901-4.
Chen L, Zhou C, Chen Q, Shang J, Liu Z, Guo Y, Li C, Wang H, Ye Q, Li X, Zu S, Li F, Xia Q, Zhou T, Li A, Wang C, Chen Y, Wu A, Qin C, Man J. Oncolytic Zika virus promotes intratumoral T cell infiltration and improves immunotherapy efficacy in glioblastoma. Mol Ther—Oncolytics. 2022;24:522–34. https://doi.org/10.1016/j.omto.2022.01.011.
Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat Rev Cancer. 2014;14(8):559–67. https://doi.org/10.1038/nrc3770.
Prestwich RJ, Harrington KJ, Pandha HS, Vile RG, Melcher AA, Errington F. Oncolytic viruses: a novel form of immunotherapy. Expert Rev Anticancer Ther. 2008;8(10):1581–8. https://doi.org/10.1586/14737140.8.10.1581.
Tang C, Li L, Mo T, Na J, Qian Z, Fan D, Sun X, Yao M, Pan L, Huang Y, Zhong L. Oncolytic viral vectors in the era of diversified cancer therapy: from preclinical to clinical. Clin Transl Oncol. 2022;24(9):1682–701. https://doi.org/10.1007/s12094-022-02830-x.
Cejalvo JM, Falato C, Villanueva L, Tolosa P, González X, Pascal M, Canes J, Gavilá J, Manso L, Pascual T, Prat A, Salvador F. Oncolytic viruses: a new immunotherapeutic approach for breast cancer treatment. Cancer Treat Rev. 2022;106:102392. https://doi.org/10.1016/j.ctrv.2022.102392.
Haseley A, Alvarez-Breckenridge C, Chaudhury A, Kaur B. Advances in oncolytic virus therapy for glioma. Recent Pat CNS Drug Discov. 2009;4(1):1–13. https://doi.org/10.2174/157488909787002573.
Hemminki O, dos Santos JM, Hemminki A. Oncolytic viruses for cancer immunotherapy. J Hematol Oncol. 2020;13(1):84. https://doi.org/10.1186/s13045-020-00922-1.
Garg AD, Dudek-Peric AM, Romano E, Agostinis P. Immunogenic cell death. Int J Dev Biol. 2015;59(1-2–3):131–40. https://doi.org/10.1387/ijdb.150061pa.
Schmidt SV, Nino-Castro AC, Schultze JL. Regulatory dendritic cells: there is more than just immune activation. Front Immunol. 2012. https://doi.org/10.3389/fimmu.2012.00274.
Couzin-Frankel J. Cancer immunotherapy. Science. 2013;342(6165):1432–3. https://doi.org/10.1126/science.342.6165.1432.
Mostafa A, Meyers D, Thirukkumaran C, Liu P, Gratton K, Spurrell J, Shi Q, Thakur S, Morris D. Oncolytic reovirus and immune checkpoint inhibition as a novel immunotherapeutic strategy for breast cancer. Cancers. 2018;10(6):205. https://doi.org/10.3390/cancers10060205.
Gholami S, Marano A, Chen NG, Aguilar RJ, Frentzen A, Chen C-H, Lou E, Fujisawa S, Eveno C, Belin L, Zanzonico P, Szalay A, Fong Y. A novel vaccinia virus with dual oncolytic and anti-angiogenic therapeutic effects against triple-negative breast cancer. Breast Cancer Res Treat. 2014;148(3):489–99. https://doi.org/10.1007/s10549-014-3180-7.
Mardi A, Shirokova AV, Mohammed RN, Keshavarz A, Zekiy AO, Thangavelu L, Mohamad TAM, Marofi F, Shomali N, Zamani A, Akbari M. Biological causes of immunogenic cancer cell death (ICD) and anti-tumor therapy; combination of oncolytic virus-based immunotherapy and CAR T-cell therapy for ICD induction. Cancer Cell Int. 2022;22(1):168. https://doi.org/10.1186/s12935-022-02585-z.
Nutter Howard FH, Iscaro A, Muthana M (2022) Oncolytic viral particle delivery. In Systemic Drug Delivery Strategies (pp. 211–230). Elsevier, https://doi.org/10.1016/B978-0-323-85781-9.00008-7
Burgess HM, Pourchet A, Hajdu CH, Chiriboga L, Frey AB, Mohr I. Targeting Poxvirus decapping enzymes and mRNA decay to generate an effective oncolytic virus. Mol Ther—Oncolytics. 2018;8:71–81. https://doi.org/10.1016/j.omto.2018.01.001.
Gal-Ben-Ari S, Barrera I, Ehrlich M, Rosenblum K. PKR: a kinase to remember. Front Mol Neurosci. 2019;11:480. https://doi.org/10.3389/fnmol.2018.00480.
Ripp J, Hentzen S, Saeed A. Oncolytic viruses as an adjunct to immune checkpoint inhibition. Front Biosci-Landmark. 2022;27(5):151. https://doi.org/10.31083/j.fbl2705151.
Li D, Wu M. Pattern recognition receptors in health and diseases. Signal Transduct Target Ther. 2021;6(1):291. https://doi.org/10.1038/s41392-021-00687-0.
Sitta J, Claudio PP, Howard CM. Virus-Based Immuno-Oncology Models. Biomedicines. 2022;10(6):1441. https://doi.org/10.3390/biomedicines10061441.
Ahrends T, Borst J. The opposing roles of CD4+ T cells in anti-tumour immunity. Immunology. 2018;154(4):582–92. https://doi.org/10.1111/imm.12941.
Marchini A, Daeffler L, Pozdeev VI, Angelova A, Rommelaere J. Immune Conversion of tumor microenvironment by oncolytic viruses: the protoparvovirus H-1PV case study. Front Immunol. 2019;10:1848. https://doi.org/10.3389/fimmu.2019.01848.
Kielbik M, Szulc-Kielbik I, Klink M. Calreticulin—multifunctional chaperone in immunogenic cell death: potential significance as a prognostic biomarker in ovarian cancer patients. Cells. 2021;10(1):130. https://doi.org/10.3390/cells10010130.
Das K, Belnoue E, Rossi M, Hofer T, Danklmaier S, Nolden T, Schreiber L-M, Angerer K, Kimpel J, Hoegler S, Spiesschaert B, Kenner L, von Laer D, Elbers K, Derouazi M, Wollmann G. A modular self-adjuvanting cancer vaccine combined with an oncolytic vaccine induces potent antitumor immunity. Nat Commun. 2021;12(1):5195. https://doi.org/10.1038/s41467-021-25506-6.
Lemos de Matos A, Franco LS, McFadden G. Oncolytic viruses and the immune system: the dynamic duo. Mol Ther—Methods Clin Dev. 2020;17:349–58. https://doi.org/10.1016/j.omtm.2020.01.001.
O’Leary MP, Warner SG, Kim S-I, Chaurasiya S, Lu J, Choi AH, Park AK, Woo Y, Fong Y, Chen NG. A novel oncolytic chimeric orthopoxvirus encoding luciferase enables real-time view of colorectal cancer cell infection. Mol Ther—Oncolytics. 2018;9:13–21. https://doi.org/10.1016/j.omto.2018.03.001.
Warner SG, Kim S-I, Chaurasiya S, O’Leary MP, Lu J, Sivanandam V, Woo Y, Chen NG, Fong Y. A novel chimeric poxvirus encoding hnis is tumor-tropic, imageable, and synergistic with radioiodine to sustain colon cancer regression. Mol Ther—Oncolytics. 2019;13:82–92. https://doi.org/10.1016/j.omto.2019.04.001.
Kim S-I, Park AK, Chaurasiya S, Kang S, Lu J, Yang A, Sivanandam V, Zhang Z, Woo Y, Priceman SJ, Fong Y, Warner SG. Recombinant orthopoxvirus primes colon cancer for checkpoint inhibitor and cross-primes T cells for antitumor and antiviral immunity. Mol Cancer Ther. 2021;20(1):173–82. https://doi.org/10.1158/1535-7163.MCT-20-0405.
O’Leary MP, Choi AH, Kim S-I, Chaurasiya S, Lu J, Park AK, Woo Y, Warner SG, Fong Y, Chen NG. Novel oncolytic chimeric orthopoxvirus causes regression of pancreatic cancer xenografts and exhibits abscopal effect at a single low dose. J Transl Med. 2018;16(1):110. https://doi.org/10.1186/s12967-018-1483-x.
Zhang Z, Yang A, Chaurasiya S, Park AK, Lu J, Kim S-I, Warner SG, Yuan Y-C, Liu Z, Han H, Von Hoff D, Fong Y, Woo Y. CF33-hNIS-antiPDL1 virus primes pancreatic ductal adenocarcinoma for enhanced anti-PD-L1 therapy. Cancer Gene Ther. 2021. https://doi.org/10.1038/s41417-021-00350-4.
Chaurasiya S, Yang A, Kang S, Lu J, Kim S-I, Park AK, Sivanandam V, Zhang Z, Woo Y, Warner SG, Fong Y. Oncolytic poxvirus CF33-hNIS-ΔF14.5 favorably modulates tumor immune microenvironment and works synergistically with anti-PD-L1 antibody in a triple-negative breast cancer model. OncoImmunology. 2020;9(1):1729300. https://doi.org/10.1080/2162402X.2020.1729300.
Zhang Z, Yang A, Chaurasiya S, Park AK, Kim S-I, Lu J, Olafsen T, Warner SG, Fong Y, Woo Y. PET imaging and treatment of pancreatic cancer peritoneal carcinomatosis after subcutaneous intratumoral administration of a novel oncolytic virus, CF33-hNIS-antiPDL1. Mol Ther—Oncolytics. 2022;24:331–9. https://doi.org/10.1016/j.omto.2021.12.022.
CF33-hNIS-antiPDL1 for the Treatment of Metastatic Triple Negative Breast Cancer—Full Text View—ClinicalTrials.gov. (n.d.) https://clinicaltrials.gov/ct2/show/NCT05081492.
A Study of CF33-hNIS (VAXINIA), an Oncolytic Virus, as Monotherapy or in Combination with Pembrolizumab in Adults with Metastatic or Advanced Solid Tumors ClinicalTrials.gov. (n.d.). https://clinicaltrials.gov/ct2/show/NCT05346484. Acccessed 23 Aug 2022,
Acknowledgements
The authors are grateful to Shoolini University for providing junior research scholarship to Simran Deep Kaur and also to all the researchers who discovered Vaxinia virus and immuno-oncolytic drug delivery system that was helpful for framing this review paper.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors were involved in the study’s conception and initiation. The paper’s outline and structure were designed by SDK. All the figures and tables in the original draft were made by SDK. DNK and ADS read and commented on earlier versions of the paper. The manuscript is edited by SDK and DNK. The manuscript was edited under DNK’s direction. The final version, which was the result of multiple rounds of editing, was approved by all of the authors.
Corresponding author
Ethics declarations
Conflict of interest
Simran Deep Kaur, Deepak N Kapoor, Aman Deep Singh declare that they have no conflict of interest.
Ethical approval and consent to participations
Not applicable.
Consent for publications
We agreed with the journal policy and provided our consent for the publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaur, S.D., Singh, A.D. & Kapoor, D.N. Current perspectives on Vaxinia virus: an immuno-oncolytic vector in cancer therapy. Med Oncol 40, 205 (2023). https://doi.org/10.1007/s12032-023-02068-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02068-9